Department of Biological & Health Sciences, Texas A & M University, Texas, USA
The plastination process using vacuum impregnation replaces tissue fluids with curable polymers and results in dry, non-toxic specimens. We detail a method to produce high quality plastinated specimens using an injection impregnation process. This alternate, non-vacuum method is very low-tech and has minimal start-up costs. We were able to successfully use this technique on small vertebrates ranging from 1 to 700 grams. The plastinated specimens were life-like and the natural contours of the animals were maintained. Dissection revealed polymer had penetrated throughout the viscera and deep muscles. In addition, internal morphology including major muscle groups retained their shape with no apparent shrinkage.
alternate process; inexpensive; injection; plastination; low-tech; small vertebrates
Randy L. Powell: Department of Biological and Health Sciences, MSC 158, Texas A&M University-Kingsville, Kingsville, Texas 78363, USA. Telephone: (361) 593-2346; e-mail: randy.powell@tamuk.edu
![]()



Plastination is a multi-step process in which tissue fluids are replaced by curable polymers; e.g., silicone, epoxy, polyester (Bickley et al., 1981; Pashaei, 2010). The results are dry, durable specimens (entire/partial body or individual organs) that look more life-like. Because plastinated specimens are non-toxic, they can be freely handled and examined without gloves. Consequently, the incorporation of plastinated specimens for teaching and research purposes has been adopted by medical and veterinary schools worldwide (Dawson et al., 1990; Cook, 1996; Mansor, 1996; Correia et al., 1998; Peris, 2000; Zhong et al., 2000).
The fundamentals of the plastination process were originally developed and documented by Gunther von Hagens over 30 years ago (von Hagens et al., 1987; see Pashaei, 2010 for a more detailed history). While the basic principles of plastination have remained somewhat consistent since its initial introduction (i.e., fixation, dehydration, forced vacuum impregnation, and curing), there have been numerous developments, alternate techniques, and improvements in the process. These developments include: new polymers that permit ambient (room) temperature plastination (Tianzhong et al., 1998; Henry, 2007; Raoof et al., 2007), progress in sheet and ultra thin sheet plastination (Latorre et al., 2004; Sora et al., 2004; Latorre and Henry, 2007; Sora, 2007), staining and re-coloring of plastinated tissue (Riepertinger and Heuckendorf, 1993; Suriyaprapadilok and Withyachumnarnkul, 1997; An and Zhang, 1999; Mendez, 2008; Steinke et al., 2008a), and methods to produce light-weight plastinated specimens (Henry and Nel, 1993; Steinke et al., 2008b).
Integrating plastinated specimens into a teaching environment has been shown to provide positive educational outcomes in the classroom (Latorre et al., 2011). The characteristics of plastinated specimens (life-like feel, no odor, no toxicity) allow students the benefit of greater tactile interaction with the specimens which may result in an increased time spent with the material. Moreover, incorporation of plastinated specimens in teaching labs has been shown as an excellent method to improve the “hands-on experience” (see Dawson et al., 1990, for a student survey of responses to plastinated specimens). Similarly, the use of plastinated specimens for exhibition and display in museums has been demonstrated as equally valuable. Unfortunately, start-up costs for the production of plastinated specimens using traditional methods may be prohibitive for small labs and institutions with limited budgets and space. Furthermore, traditional plastination methods require special safety consideration and equipment, such as explosion hazards and adequate exhaust venting (Henry and Nel, 1993). Plastinated specimens (entire/partial body forms and individual organs) are available for purchase from a few companies; however, availability is limited to a small number of species, and the specimens can be quite expensive.
Vacuum impregnation, which allows for the penetration and saturation of curable polymers into tissue, has been the hallmark step in plastination. While injection (i.e., into deep tissues on large specimens or into specialized structures) has been suggested to improve silicone perfusion during vacuum impregnation (Henry and Nel, 1993; Sivrev et al., 1997), it has not been reported for use exclusively as a mechanism for polymer introduction. Although large organisms may not be good candidates, smaller specimens, with less tissue mass, can be thoroughly saturated with polymer using injection alone. Plastination of small organisms has received little attention, and scant information has been reported (see Asadi and Mahmodzadeh, 2004). Heretofore, no one has reported on injection impregnation for small, whole-body specimens. Our goals were to produce high quality plastinated specimens using an alternate, non-vacuum impregnation process, and to minimize start-up costs.
Fixation: Specimens were fixed in 10% formalin (3.7% absolute formaldehyde) by injection using oral and cloacal injection sites with 21G-18G needles. Following injection, specimens were positioned for hardening and submerged in 10% formalin at room temperature for 24 to 48 hours. After fixation, specimens were rinsed thoroughly in tap water to remove any excess, unbound formalin.
Dehydration: Specimens were submerged in three consecutive 100% acetone baths at room temperature with a 1:10 specimen/acetone ratio. In addition, the specimens were flushed through the previous injection sites (using a syringe and 21G needle) with 100% acetone initially and between baths. Each bath lasted two to five days, and the specimen/acetone mixture was agitated once each day. Acetone concentration was monitored with a hydrometer to determine the progression of specimen dehydration. Dehydration was considered satisfactory when the final acetone bath reached a specific gravity of 0.80.
Silicone impregnation: Specimens were removed from the final acetone bath, and a silicone polymer mixture was immediately applied to the entire surface of the specimens using a brush. Following surface application, specimens were injected with silicone polymer mixture (via previous injection sites, penetrating into body cavities and deep tissue) with 23G and 21G needles and 1 to 5 ml syringes. Larger specimens that required more silicone (e.g., large rodents), were injected using 50 ml syringes and 18G or 15G needles. The amount of polymer injected into the specimens varied between 1 and 25 ml per injection site depending on the size and species of the organism. The objective was to force in as much silicone as possible (until it began to leak out), while maintaining the original morphology of the specimen. The silicone polymer mixture was composed of NCS10 and the cross-linker, NCS6 (North Carolina products) at a ratio of 90:10. The NCS10/6 mixture remains stable at room temperature (Henry, 2007) and has a very low viscosity (easily injected through 23G needles with a 1 ml syringe). After the initial surface application and injection, specimens were wrapped in a layer of paper towels followed by an external layer of thin plastic wrap (to slow acetone evaporation).
Specimens were inspected daily, injected with additional NCS10/6 mixture (to replace evaporating acetone), and re-wrapped in clean paper towels and plastic wrap. Daily injection with the NCS10/6 mixture continued until no acetone odor was detected on the paper towels or the specimen (approx. 3 to 15 days). After acetone evaporation was complete, the specimens were injected with NCS10/6/3 mixture of 90% NCS10/6 and 10% NCS3 (catalyst: North Carolina products), followed by a thin surface application (with a small brush) of NCS3 on the entire specimen. Mammalian specimens required blotting to remove excess NCS10/6 from the fur. The catalyst (NCS3) was then applied directly to the skin at various points using a 1ml plastic syringe to limit polymerization in the fur. The specimens were covered with thin plastic wrap. Daily inspection and additional NCS10/6/3 mixture injection was repeated as necessary for three to five days.
Curing: Specimens were unwrapped, wiped clean of any silicone polymer that may have leaked out, and placed on paper towels in a small aquarium. Approximately 1ml of NCS5 (chain extender: North Carolina products) was placed in an open (60 X15mm) cell culture dish and a glass cover was placed over the aquarium to maintain an atmosphere of NCS5 vapor. Each subsequent day, chain extender (NCS5) was replaced, the specimens were wiped clean of any uncured silicone polymer that may have leaked, and specimens were inspected for any signs of distortion (shrinkage of the tail, legs or abdominal areas). If necessary, additional NCS10/6/3 polymer mixture was injected at or near any distorted areas. Excess polymer was periodically brushed from the pelage of mammals during the entire curing time. Specimens were maintained in the NCS5 vapor chamber for final curing for 20 to 30 days.
Numerous vertebrate species that included an assortment of amphibians, reptiles, and mammals were plastinated. Specimens ranged in size from a 1.0g lizard to a 700g snake. The plastinated specimens were odorless, their surfaces were dry to the touch, and they exhibited a small degree of flexibility. In general, the plastinated specimens were life-like and the natural contours of the animals were maintained (Figs. 1, 2, 3). Gross dissection was performed on several specimens. Silicone had penetrated throughout the viscera and the internal morphology was maintained, as major organs remained in approximate position (Figs. 4, 5). The internal structures were odorless and dry with negligible shrinkage. Superficial, as well as deep muscles appeared thoroughly plastinated and major muscle groups retained their shape with little to no reduction in size (Fig. 6). Histological examination revealed polymer perfusion into the tissues of the internal organs and muscles, and that polymer had filled the interstitial as well as intracellular spaces.
 Figure 1: Various species of mammals, amphibians and reptiles were plastinated using the injection method. In general, natural contours of the animals were maintained. a) Western Diamondback Rattlesnake, Crotalus atrox, b) Texas Coralsnake, Micrurus tener, c) Hispid Cotton Rat, Sigmodon hispidus, d) Mexican Spiny Pocket Mouse, Liomys irroratus, e) Yellow Mud Turtle, Kinosternon flavescens, f) Brazilian Free-tailed Bat, Tadarida brasiliensis, g) Cane Toad, Rhinella marina, h) Texas Toad, Anaxyrus speciosus, i) Prairie Skink, Plestiodon septentrionalis. |
 Figure 2: Texas Coralsnake (Micrurus tener). This specimen displayed excellent color retention. |
 Figure 3: Cane Toad (Rhinella marina). The natural contours of the animal were well maintained. Glass eyes were installed on this specimen to enhance its life-like appearance. |
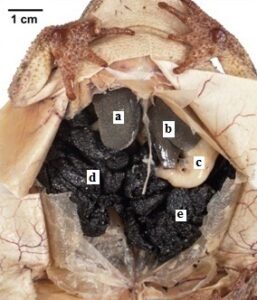 Figure 4: Gulf Coast Toad (Bufo nebulifer). The specimen shows polymer penetration of the viscera and preservation of organs and internal morphology. a, b) liver, c) stomach, d, e) egg mass |
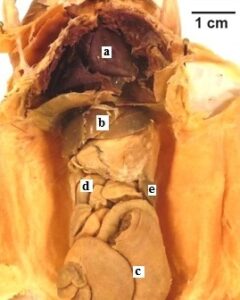 Figure 5: Hispid Cotton Rat (Sigmodon hispidus). The specimen shows polymer penetration of the viscera and preservation of internal morphology. a) heart, b) liver, c) cecu, d) small intestine, e) spleen. |
 Figure 6. Hispid Cotton Rat (Sigmodon hispidus). The muscles of this specimen are thoroughly plastinated and muscle groups retained morphology. |
We argue that the success of the injection method is based on the physical properties and interactions of animal integument, acetone, and silicone polymer. Injection of silicone polymer results in an increased internal pressure that is contained by the integument of the specimen. The semi-permeability of the integument allows the passage of acetone molecules, while retaining the larger, more viscous silicone molecules. The much heavier silicone polymer (~27,000 g mol-1) readily displaces the lighter acetone molecules (58.08 g mol-1), causing movement of acetone through the integument, mouth, and cloaca. Acetone rapidly evaporates at room temperature; however, silicone polymer has an extremely low vapor pressure and negligible evaporation. In addition, the degree of keratinization of the integument results in varying rates of evaporation due to differences in permeability. These differences necessitate special measures for less keratinized specimens, such as amphibians, which require rapid coating with NCS10/6 and wrapping, to slow acetone evaporation. It should be emphasized that premature evaporation of acetone causes pronounced shrinkage and stiffness in specimens and prevents adequate injection of silicone polymer resulting in plastinated specimens of poorer quality.
The results demonstrate that high-quality plastinated specimens can be produced using only injection impregnation. This alternate, non-vacuum process is very low-tech and has minimal start-up costs. Injection plastination will allow small laboratories on limited budgets the ability to process specimens for teaching collections and display. Although we were able to use this technique successfully on a 700g animal, there are no doubt limitations to the upper size of specimens that can be successfully processed with this method. It should be emphasized that to produce high-quality plastinated specimens, it is important to be consistent with daily inspection and processing of specimens; especially in controlling the rate of acetone evaporation and adequate injection of silicone polymer. As with traditional plastination techniques, there is a small learning curve and some degree of skill and art involved. Our preliminary findings also indicate that small animal plastinated specimens are better teaching models in taxonomic lab courses and are preferred over traditional fluid-preserved and dry prepared specimens.
Acknowledgements
The following people were helpful in providing suggestions, helpful information, and/or feedback: Dr. Robert W. Henry, College of Veterinary Medicine, The University of Tennessee; Faculty, staff, and students of mammalogy and vertebrate zoology from the Department of Biological and Health Sciences, Texas A&M University-Kingsville.
An P, Zhang M. 1999: A technique for preserving the subarachnoid space and its contents in a natural state with different colours. J Int Soc Plastination 14(1): 12-17.
https://doi.org/10.56507/CQUW3856
Asadi MH, Mahmodzadeh A. 2004: Ascaris plastination through S10 techniques. J Int Soc Plastination 19: 20-2.
https://doi.org/10.56507/EDVF4592
Bickley HC, von Hagens G, Townsend, FM. 1981: An improved method for the preservation of teaching specimens. Arch Pathol Lab Med 105: 674-6.
Cook P. 1996: Plastination as a clinically based teaching aid at the University of Auckland. Abstract presented at The 8th International Conference on Plastination - University of Queensland, Brisbane, Australia. July 1996. J Int Soc Plastination 11: 22.
Correia JAP, Prinz RAD, Benevides de Freitas EC, Pezzi LHA. 1998: Labeling and storing plastinated specimens-An experience from Universidade Federal Do Rio De Janeiro. J Int Soc Plastination 13(2): 17-20.
https://doi.org/10.56507/LTAD6864
Dawson TP, James RS, Williams GT. 1990: Silicone plastinated pathology specimens and their teaching potential. J Pathol 162: 265-72. https://doi.org/10.1002/path.1711620314
https://doi.org/10.1002/path.1711620314
Henry RW. 2007: Silicone plastination of biological tissue: Room temperature technique North Carolina technique and products. J Int Soc Plastination 22: 26-30.
https://doi.org/10.56507/FSNZ3092
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7: 27-31.
https://doi.org/10.56507/WUXP9436
Latorre R, Arencibia A, Gil F, Rivero M, Ramirez G, Vaquez-Auto JM, Henry RW. 2004: Sheet plastination with polyester: An alternative for all tissues. J Int Soc Plastination 19: 33-39.
https://doi.org/10.56507/OFGF7088
Latorre R, Henry RW. 2007: Polyester plastination of biological tissue: P40 technique for body slices. J Int Soc Plastination 22: 69-77.
https://doi.org/10.56507/CARV3913
Latorre RM, García-Sanz MP, Moreno M, Hernández F, Gil F, López O, Ayala MD, Ramírez G, Vázquez JM, Arencibia A, Henry RW. 2011: How useful is plastination in learning anatomy? J Vet Med Ed 34: 172-176.
https://doi.org/10.3138/jvme.34.2.172
Mansor O. 1996: Use of plastinated specimens in a medical school with a fully integrated curriculum. J Int Soc Plastination 11: 16-17.
https://doi.org/10.56507/MMLW7935
Mendez BA, Romero RL, Trigo FJ, Henry RW, Candanosa AE. 2008: Evaluation of imidazole for color reactivation of pathological specimens of domestic animals. J Int Soc Plastination 23: 17-24.
https://doi.org/10.56507/HZTR8339
Pashaei S. 2010: A brief review on the history, methods and applications of plastination. Int J Morphol 28: 1075-1079.
https://doi.org/10.4067/S0717-95022010000400014
Peris KJ. 2000: Plastination technology for biomedical research and studies in Kenya. J Int Soc Plastination 15: 4-9.
https://doi.org/10.56507/OQRJ8580
Raoof A, Henry RW, Reed RB. 2007: Silicone plastination of biological tissue: Room temperature technique Dow™/Corcoran technique and products. J Int Soc Plastination 22: 21-25.
https://doi.org/10.56507/AWAC9285
Riepertinger A, Heuckendorf E. 1993: E 20 color-injection and plastination of the brain. J Int Soc Plastination 7: 8-12.
https://doi.org/10.56507/YHON8469
Sivrev D, Kayriakov J, Trifonov Z, Djelebov D, Atanasov M. 1997: Combined plastination methods for preparation of improved ophthalmologic teaching models. J Int Soc Plastination 12(2): 12-14.
https://doi.org/10.56507/YCNS8311
Sora MC. 2007: Epoxy plastination of biological tissue: E12 ultra-thin technique. J Int Soc Plastination 22: 40-45.
https://doi.org/10.56507/TQMH6049
Sora MC, Strobl B, Radu J. 2004: High temperature E12 plastination to produce ultra-thin sheets. J Int Soc Plastination 19: 22-25.
https://doi.org/10.56507/NOQQ3899
Steinke H, Rabi S, Saito T. 2008a: Staining body slices before and after plastination. Eur J Anat 12: 51-55.
Steinke H, Rabi S, Saito T, Sawutti A, Miyaki T, Itoh M, Spanel-Borowski K. 2008b: Light-weight plastination. Ann Anat 190: 428-431.
https://doi.org/10.1016/j.aanat.2008.02.005
Suriyaprapadilok L, Withyachumnarnkul B. 1997: Plastination of stained sections of the human brain: Comparison between different staining methods. J Int Soc Plastination 12(1): 27-32.
https://doi.org/10.56507/YISQ6047
Tianzhong Z, Jingren L, Kerming Z. 1998: Plastination at room temperature. J Int Soc Plastination 13(2): 21-25.
https://doi.org/10.56507/YSHV9792
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175: 411-21.
https://doi.org/10.1007/BF00309677
Zhong ZT, Xuegui Y, Ling C, Jingren L. 2000: The history of plastination in China. J Int Soc Plastination 15: 25-29.
https://doi.org/10.56507/QSNK3285