Division of Biomedical Sciences (Anatomy), St George's, University of London, London, UK
Objectives
The medial wall of the orbit is reported to contain anterior and posterior ethmoidal foramina, through which pass branches of the ophthalmic artery. These arteries are a potential source of bleeding during surgical procedures involving the medial orbital wall. However, recent research has revealed variable numbers of accessory ethmoidal foramina, which have also been shown to transmit vascular structures, making intraorbital surgery unpredictable and potentially hazardous. This study aims to elucidate the branching pattern of the arterial supply of the medial orbital wall, particularly in cases of multiple ethmoidal foramina.
Materials and Methods
Orbits were retrieved from cadavers donated for anatomical examination. Red silicone was injected into the ophthalmic artery via the internal carotid. The medial orbital wall was then dissected from contiguous craniofacial structures and embedded in Biodur® Epoxy E12 resin. Sections of 0.3 mm thickness were cut with a slow speed diamond saw, stained with Miller’s stain for elastin and then photographed with a digital camera. Three-dimensional reconstructions were carried out using WinSURF software.
Results
The optical qualities of the epoxy resin blocks were excellent, though this was not always the case with the individual sections. However, in the stained sections, the arteries were clearly visible. Using WinSURF, the outlines of the branches of the ethmoidal arteries and the bone lining the medial wall of the orbit were delineated. A three-dimensional model of the pattern of arterial branching was created.
Conclusion
Surgeons operating along the medial wall of the orbit need to be aware that multiple branches of the ethmoidal artery may be encountered. Three-dimensional reconstructions of the branching pattern give a clearer understanding of the blood supply to the medial wall. Work is on-going to map the variations in the branching of the ophthalmic artery.
ethmoidal arteries; ethmoid foramen; plastination; epoxy; 3-D reconstruction
Philip J Adds, Institute of Medical and Biomedical Education (Anatomy), St Georges, University of London, Cranmer Terrace, London SW17 0RE, UK. Telephone +44(0) 2087255208, email padds@sgul.ac.uk
![]()



The medial wall of the orbit and its arteries are of much interest to ophthalmic and oculoplastic surgeons, who may need to carry out procedures involving the medial orbital wall in cases of trauma, severe epistaxis or thyroid eye disease. It is vital, therefore, that the surgeon operating in this region has access to a full and accurate description of the vascular structures that they are likely to encounter.
Conventional wisdom describes the medial orbital wall as being formed by contributions from the ethmoid, maxilla and lacrimal bones, with the body of the sphenoid posteriorly, and pierced by two foramina (the anterior and posterior ethmoidal foramina) that lie along the fronto-ethmoidal suture line (Fig. 1).
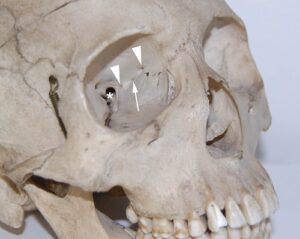
Figure 1. Skull showing the medial wall of the orbit. Arrowheads indicate anterior and posterior ethmoidal foramina, * indicates the optic canal. A single accessory foramen can be seen between the anterior and posterior foramina (arrow).
The bone of the ethmoid is particularly thin (0.2 – 0.4 mm in thickness) and is consequently very vulnerable to traumatic or iatrogenic fracture. The lamina papyracea so formed makes up the majority of the surface of the medial orbital wall (Dutton, 2011).
The anterior and posterior ethmoidal foramina are important landmarks during surgical exploration along the medial orbital wall, and their relative positions are often remembered by the “24-12-6” rule. This describes the distance in millimeters between the anterior lacrimal crest and the anterior ethmoidal foramen, from the anterior ethmoidal foramen to the posterior ethmoidal foramen and finally from the posterior ethmoidal foramen to the optic canal (Abed et al., 2012).
The ethmoidal foramina transmit important neurovascular structures. The anterior and posterior ethmoidal arteries, branches from the ophthalmic artery, pass through the anterior and posterior foramina respectively. The ophthalmic artery is the first branch from the internal carotid artery as it enters the middle cranial fossa (Fig.2). The nasociliary nerve, a branch of the ophthalmic division of the trigeminal, gives off anterior and (variably) posterior ethmoidal branches that accompany the arteries through the respective foramina to supply the ethmoidal air cells, anterior cranial cavity and (via the cribriform plate), the nasal cavity (Dutton, 2011).
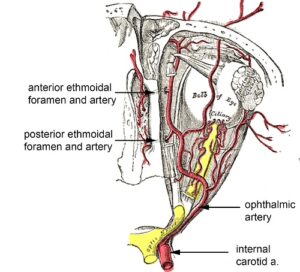
Figure 2. Conventional depiction of the anterior and posterior ethmoidal arteries passing through their respective foramina. Adapted from Bartleby.com.
It is well known that the position, and indeed number, of the ethmoidal foramina is likely to vary between individuals, and recently the degree of variation has been assessed in both Asian and Caucasian orbits. Takahashi et al. (2011) examined 54 Japanese orbits and reported either one or two extra accessory ethmoidal foramina in 18 orbits (33.3 %), while Abed et al. (2012), in a study of 47 Caucasian orbits, reported variations (including the first report in the literature of quintuple foramina) in 21 orbits (44%), and furthermore suggested that the navigational aide memoire should be revised to “26-17-7”.
The question remained to be answered whether these accessory foramina, occurring in nearly half the Caucasian and a third of the Asian population were likely to be of significance. Are these accessory foramina merely defects in the bony wall of the orbit, or do they, like the anterior and posterior foramina, also transmit vascular (and possibly neural) structures? Anecdotal evidence from ENT and ophthalmic surgeons suggests that unexpected bleeding is often encountered, and a histological study carried out by the authors using a dissecting microscope suggests that, not only are there many more accessory foramina that previously suspected (8 were identified in one individual), but they all transmit vascular structures (data not shown). The text-book image of the medial wall, then, is clearly in need of revision. The distribution and branching pattern of the ethmoidal arteries in one-third to one-half of the population is likely to be significantly more complex than previously suspected.
In this study, we aimed to reconstruct the 3-D branching pattern of the ethmoidal arteries using WinSURF software, (www.akuaware.com, Kailua, HI, USA), by embedding the medial orbital wall in Biodur® E12 resin and cutting thin sections which were then photographed. The method used was adapted from the standard published E12 ultra-thin epoxy technique described by Sora (2007).
Specimen preparation
Pairs of orbits were retrieved from fresh-frozen cadaveric heads in the Dissecting Room at St George’s School of Medicine, London, UK. The cadavers had all undergone serological testing and appropriate consent had been obtained prior to death. Prior to further dissection, the inferior (proximal) end of the internal carotid artery was clamped, and saline was pumped through the superior end using a syringe and cannula to flush the arteries. Low-viscosity red silicone resin (Biodur® KEM 06) was then injected into the ophthalmic artery via the internal carotid, using a 1 ml syringe with a wide-bore cannula.
The skull section was then immersed in 4% formalin for one week to fix the tissues before further dissection was carried out. The specimen was removed from the formalin, drained and rinsed under running tap water. The skin was removed from the lateral aspect of the skull, then using a hand-held oscillating saw (de Soutter Medical), the lateral half of the orbits was removed and the two orbits were split down the mid-line. The globe, lateral ocular adnexa and periorbital fat were removed by careful dissection to expose the medial extra-ocular muscles (superior oblique and medial rectus) and the ophthalmic artery and its branches (Fig. 3).
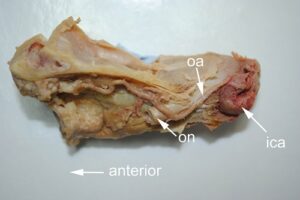
Figure 3. Left orbit section (viewed from lateral side); ica, internal carotid artery; oa, ophthalmic artery; on, optic nerve.
Dehydration and impregnation
The dissected specimen was dehydrated by immersion in 100% acetone (VWR) pre-cooled to -20° and stored in a sealed container in the freezer. The acetone was replaced daily for three days with fresh 100% pre-cooled acetone, after which the container was brought out of the freezer and allowed to equilibrate to room temperature before the specimen was transferred to fresh acetone at room temperature to dissolve the fat.
For impregnation, a mixture of fresh Biodur® E12/E6/E600 was prepared using the ratio by volume of 100/50/0.2, and mixed thoroughly. The dissected orbit was submerged in the mixed resin and placed in a vacuum oven at 30° C (Heraeus VT 6130 M), where it was allowed to equilibrate overnight. The pressure was then lowered (i.e. the vacuum was increased) in stages as follows: 40 cm Hg (550 Mbar), 32 cm Hg (425 Mbar), 24 cm Hg (320 Mbar), 16 cm Hg (210 Mbar) for 45 minutes to 1 hour at each stage, before the temperature was raised to 60° C for the final 2 stages: 8 cm Hg (100 Mbar), 2 cm Hg (25 Mbar). The temperature was raised for the final two stages in order to decrease the viscosity of the resin and thereby facilitate penetration of the resin into the specimen. Using this protocol, vacuum impregnation can be completed in one day following the overnight incubation.
Embedding, sectioning and staining
The specimen was removed from the vacuum and transferred to a mold for embedding. The molds were cut out of blocks of Styrofoam, lined with kitchen foil (Fig. 4); small plastic food containers are also ideal for this
purpose. If Styrofoam is used, great care must be taken to avoid overfilling, as spillage of the resin will dissolve the Styrofoam. The same resin mixture that was used for the impregnation stage can be used for embedding; it is not necessary to prepare a fresh mixture since the impregnation stage is so short. The mold was then placed in the oven at 65° C for 5 days to harden. It may be helpful to indicate anterior/posterior and medial/lateral on the mould with a permanent marker before placing in the oven.
Before sectioning, the block can be trimmed if necessary using the oscillating saw. The trimmed block was then clamped in a Buehler Isomet slow-speed diamond saw, with a 0.4 x 127 mm blade (Fig. 5), lubricated with “Cool 2” coolant /lubricating fluid (Buehler). Serial sections of 0.3 mm thickness were cut, and the sections were washed with distilled water, dried and placed on a fibre-optic light source (Schott) and photographed with a Nikon D3100 DSLR fitted with a Sigma 105mm F2.8 macro lens (Fig. 6).
Visibility of the vascular structures can be improved by staining the sections. Epoxy-embedded thin sections can be stained directly, without the need to re-hydrate the specimens before staining. To enhance the visibility of the sectioned arteries in this study, a modified staining procedure with Miller’s stain for elastin was used:
(Modified from: http://www.ihcworld.com)
Reconstruction was carried out using WinSURF software. Objects of interest (the ophthalmic artery and its ethmoidal branches and the bone of the lamina papyracea) were identified and highlighted on the images of sequential sections.
The specimens were thoroughly impregnated despite the shortened time scale.
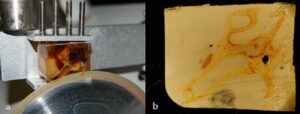
Figure 7. a) Embedded specimen being cut, showing the exceptionally good optical qualities of the epoxy block. The anterior ethmoidal artery can be clearly seen branching from the ophthalmic artery and passing through the anterior ethmoidal foramen; b) cut section before staining showing saw marks and discolouration.
The optical qualities of the epoxy resin block were excellent (Fig. 7a), though this was not always the case with the individual sections where saw-marks and discolouration affected the appearance of some of the sections (Fig. 7b). It is helpful to use frequent changes of lubricant, since the lubricant bath soon becomes clogged with residue from sawing and leads to sections with poor optical quality. Approximately 30 slices were obtained from each specimen.
Staining with Miller’s stain for elastin enhanced the visibility of the arteries by highlighting the artery walls seen in section (Fig. 8), facilitating the 3-D reconstruction.
Three-dimensional reconstruction was carried out using WinSURF 4.2 software (Moody and Lozanoff, 1997). The bone of the medial wall was easily identifiable in the sections, and the visibility of the artery sections was enhanced by histological staining with Miller’s stain for elastin. A 3-D reconstruction was created, showing the medial wall, the course of the main branch of the ophthalmic artery, and the anterior and posterior ethmoidal arteries (Fig. 9).
The reconstructed images appeared relatively well defined and bore a close resemblance to the embedded specimens. The reconstructed structures, which included the medial wall and the ophthalmic artery and its branches, can then be displayed individually or as a whole interactive model that can be rotated in 3D space and viewed in the WinSURF viewer (Fig. 9). Additionally, various features such as the transparency, colour, animation and a variety of other options can be adjusted to facilitate the visualization of the complex orbital anatomy. The resulting 3-D rendered images display details that are likely to be of considerable interest to oculoplastic or ophthalmic surgeons, permitting them to view the structures from all angles and to view their spatial relationships.
It is necessary to cut the sections have as thin as possible (0.3mm) in order to maximise the amount of information and enable an accurate reconstruction of such a small specimen. The unavoidable loss of material from the thickness of the blade (0.4 mm) meant that the total thickness of each cut was 0.7 mm, so that, inevitably, some information was lost with every cut. This disrupted the continuity of the segments, although with reasonable deduction the problem could be solved, and the 3-D reconstructions were found to give a good graphical representation of the branching pattern of the arteries. However, it may be the case that very small branches of the artery can be completely lost between sections.
Although the E12 process is time-consuming, it was found to produce sections that preserved considerable details of blood vessels, soft tissue and bone. The structure and spatial relationship of the tissues is not altered in the process. The optical qualities of the embedded blocks are excellent, and even though this was not always the case for the individual sections, it was still possible to identify the structures of interest.
Another advantage of the E12 ultra-thin technique is that it does not require decalcification of bone, and histological staining can be carried out directly on the cut sections without the need to take the sections to water beforehand, as is the case in wax-embedded sections.
In conclusion, the E12 method is an effective method for visualising details of small areas of anatomical interest. Of course, in the case of the ethmoidal arteries, where there has been found to be variation in more than 30% of individuals, it will be necessary to carry out multiple reconstructions on a large sample of orbits in order to visualise the variations in the pattern. Now that the embedding and sectioning technique has been refined, work is on-going to build up a catalogue of ethmoidal artery variants.
Acknowledgements
The authors are grateful to the donors and their families, without whose generosity this work would not have been possible. We also gratefully acknowledge the help of Ray Moss and Maria McGlyn, The Image Resource Facility, St George’s, University of London, for their invaluable assistance with the histology.
Abed SF, Shams P, Shen S, Adds PJ. Uddin, JM. 2012: A cadaveric study of ethmoidal foramina variation and its surgical significance in Caucasians. Br J Ophthalmol 96: 118-121.
https://doi.org/10.1136/bjo.2010.197319
Dutton JJ. 2011: Atlas of clinical and surgical orbital anatomy 2nd ed. Elsevier Saunders p 16-17, 87-88.
http://www.bartleby.com/107/illus514.html (accessed 29/07/2014)
http://www.ihcworld.com/_protocols/special_stains/miller%27s_elastic_ellis.htm (accessed 29/07/2014)
Moody D & Lozanoff S. 1997: SURFdriver: A practical computer program for generating three-dimensional models of anatomical structures using a PowerMac. Clin Anat 11: 132.
Sora M-C. 2007: Epoxy plastination of biological tissue: E12 ultra-thin technique. J Int Soc Plastination 22: 40-45
https://doi.org/10.56507/TQMH6049
Takahashi Y, Kakizaki H, Nakano T. 2011: Accessory ethmoidal foramina: an anatomical study. Ophthal Plast Reconstr Surg 27: 125-127.
https://doi.org/10.1097/IOP.0b013e318201c8fd