1Institute of Medical and Biomedical Education (Anatomy), St Georges, University of London London, UK.
2Adnexal Service, Moorfields Eye Hospital, London, UK
The aim of this study was to develop a method of visualising the septa that divide the retrobulbar fat body in the human orbit. Epoxy resin embedding has previously been used to visualise the branching pattern of the intra-orbital arteries. Creating a 3D reconstruction of the orbital septa would elucidate the detailed structure and orientation of the fat septa, giving valuable information to the ophthalmic surgeon.
Formalin-fixed human orbits were dissected, decalcified, dehydrated in acetone at -20° C, and impregnated with Biodur® E12 epoxy resin. Sections of 0.3 mm thickness were cut with a slow-speed diamond saw. The sections were then stained for elastin and collagen, and photographed with a digital SLR camera. The images were then used to create a 3D digital reconstruction of the fat septa using ‘Reconstruct’, a free editor for serial section microscopy. A number of different staining methods were trialled.
Lillie’s modified haematoxylin and eosin, Lillie’s trichrome, and modified Miller’s elastin stain were found to be unsuitable. Gomori’s trichrome was found to give the clearest visualisation of the soft tissues, and permitted the structures to be traced in order to create a 3-D image. However, timings had to be extended to allow the stains to penetrate the sections, and final colors were not always as expected.
Embedding in E12, combined with a Gomori’s trichrome stain is a repeatable method to visualise the architecture of the retrobulbar fat septa. A digital 3D model was created from the stained sections allowing individual structures to be isolated and manipulated. The 3D model can be used to study the morphology of the orbital fat septa in detail.
orbital fat; retrobulbar septa; epoxy embedding; plastination; epoxy; 3-D reconstruction
Philip J Adds, Institute of Medical and Biomedical Education (Anatomy), St Georges, University of London, Cranmer Terrace, London SW17 0RE, UK. Telephone +44(0) 2087255208, email padds@sgul.ac.uk
![]()



As well as providing bony protection for the eyeball, the orbit contains soft tissue structures that provide the dynamic support required for controlled eye locomotion. The primary role of the orbital fat is to help support the globe, and also to act as a shock absorber, reducing damage during trauma. Its role as an energy store is secondary, as the volume of the fat body remains constant except in cases of extreme starvation (Bremond-Gignac et al., 2004; Gaudiani et al., 2012).
The distribution of fat within the orbit however, is not uniform: it is divided into compartments by fibrous septa. The septa contain nerves and blood vessels, as well as providing a framework for the orbital fat lobules. They also provide a pulley system, connecting the extra-ocular muscles (EOMs) to each other and to the orbital periosteum (Koornneef, 1988), and there is evidence that some septa may contain smooth muscle that may play a part in binocular alignment (Miller et al., 2003) (Fig. 1).
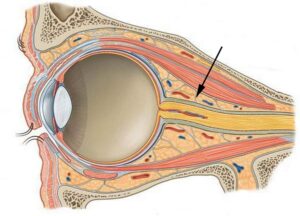
Figure 1. Diagram of sagittal section through human orbit. Arrow indicates retrobulbar fat and septum. (Modified from: Orbital connective tissue and fat. http://clinicalgate.com/the-orbit-and-accessory-visual-apparatus)
Restriction of locomotion is present in a significant number of patients following orbital decompression surgery due to muscular interactions (Richter et al., 2007). This is due to the fact that removal of the connective tissue causes an impairment in the function of the EOMs by taking away their supportive structure (Koornneef and Zonneveld, 1985)
The intricacies of the distribution and make-up of the fat septa, how they contribute to fine eye movement and how they are linked to loss of function post-ophthalmopathy and trauma are not well understood. This is mainly due to the fact that their complex branching structure is very difficult to visualise.
The epoxy resin E12 is hard, transparent and has excellent optical qualities. E12 plastination has been used previously to study the connective tissue of the spine (Johnson and Barnett, 2005), the ankle (Sora et al., 2004), and the pelvis (Sora, 2007). The hardness of the epoxy-embedded tissues enables slices less than 1 mm thick to be cut, retaining much structural detail, and permitting 3-D reconstructions to be made (Sora, 2007).
E12 resin-embedding of the human orbit has also been used successfully to visualise the path of the ethmoidal arteries, and to create a 3D model (Adds & Al-Rekabi, 2014). In their study, Adds and Rekabi (2014) cut sections 0.3 mm thick that were then stained directly; in the study reported here, we followed the same method to visualise the retrobulbar connective tissues.
The human orbit specimens were taken from cadavers donated to the Anatomy Department of St. George’s, University of London, U.K. Consent for anatomical examination and research had been given under the Human Tissue Act (2004). Nine orbits were used, from 6 cadavers. There were 4 females and 2 males, age range 67 – 97 years. None of the cadavers showed any sign of orbital pathology.
The orbits were removed from the cadaver and separated into left and right with a bandsaw. Dissection was then carried out to remove excess bone and soft tissue, leaving the orbital walls intact.
1. Decalcification.
Prior to dehydration, the individual orbits were decalcified by immersion into 20x their volume of 10% formic acid. The end point of decalcification was assessed by weighing the specimens before and during the calcification process (Mawhinney et al., 1984). A 5% loss of weight was chosen as the end point, so that the soft tissue did not become damaged due to prolonged immersion in acid. This took approximately 72 hours.
2. Dehydration.
Each orbit was then immersed in 10x its volume of 100% acetone. The acetone was pre-cooled to -20°C prior to immersion of the specimen. The pots containing the specimens and acetone were then sealed and placed in a freezer at -20°C.
The acetone was replaced twice with 72 hours between each change. Total dehydration time was 6 days. After the last change the specimens were removed from the freezer and allowed to equilibrate at room temperature. Specimens were left in room temperature acetone for a further 24 hrs for degreasing.
3. Impregnation
The impregnation resin was a mixture of Biodur® E12 resin, E6 hardener and E600 accelerator. The ratio of each of the components used was 100 : 50 : 0.2 respectively (Sora 2007).
The specimens were submerged in the resin and placed into a Heraeus vacuum oven at 30 °C for 24 hrs. No vacuum was applied for this period to allow the resin to equilibrate. After 24 hrs the pressure was decreased every hour to 550 Mbar, 425 Mbar, 320 Mbar and 210 Mbar, for the first 4 hours respectively.
For the final 2 hours of impregnation the temperature was increased to 60°C while the pressure was once again reduced to 100 Mbar and then 25 Mbar. The viscosity of the impregnation mixture has an inverse relationship with temperature meaning that for the final two hours when the temperature is increased to 60°C, its viscosity decreases enabling the resin to penetrate the specimen more effectively. During the final hour the resin impregnation mixture could be seen to bubble and splash violently, as a result of its decreased viscosity.
4. Curing
Once impregnation was complete the specimen was submerged in freshly made epoxy mixture in a plastic mould. If full impregnation has been achieved, the specimen should sink to the bottom of the container. The mould was then placed into an oven at 65 °C for at least 6 days, being checked every day, until the epoxy resin had set. Once the block had hardened it was removed from the oven and left to cool to room temperature. The block can then be removed from the plastic mould (Fig. 2).

Figure 2. A right orbit following impregnation and curing. The resin block has been removed from the plastic mould. Note the excellent optical qualities of the epoxy resin.
5. Sectioning
The resin block was first trimmed with a bandsaw to a final size of approximately 35 x 35 x 60 mm then clamped in a Buehler Isomet slow-speed saw with a 0.4 mm x 127 mm circular diamond blade. The blade was cooled with Buehler ‘Cool 2’ lubricant during cutting to avoid heat damage during sectioning. Sequential sagittal sections of 0.3 mm were cut; each section was rinsed with distilled water, blotted dry and numbered with a pencil.
6. Staining
Histology staining trials were carried out on individual sections, with the following stains:
Lillie’s modified haematoxylin and eosin (Lillie 1954), Lillie’s trichrome (Lillie’s Trichrome for muscle and collagen, 2005), modified Miller’s elastin stain (Miller, 1971), and modified Gomori’s trichrome (Gomori, 1950; IHCWORLD, 2003a, b, c). The stained sections were examined for color differentiation and clarity. The Gomori’s trichrome was found to give the best results (see Appendix for details).
The sections were stained for 1 hour, 4 times the recommended time. This was in order to allow penetration into the section. Fresh stain should be made up for each batch of sections, to preserve the strength and intensity of the stain. After staining, the sections were mounted on to HiQA™ super mega plain microscope slides using Cargille™ type BF immersion oil.
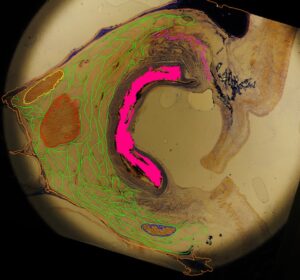
Figure 3. Sagittal section of the orbit showing colored traces: collapsed globe (pink), superior rectus (purple), superior oblique (yellow), medial rectus (red), inferior oblique (blue) and the retrobulbar fat septa (green)
7. Imaging
Digital images of each of section were taken with a Nikon D3100 DSLR camera fitted with a Sigma 105mm F2.8mm macro lens. The camera was mounted on a camera stand above a fixed light source. A steel ruler was included in the first image of each batch to act as a calibration reference when scaling the 3D model.
8. Image processing
After photographing, the brightness and contrast of each image was adjusted using Windows photo editor so that
all the fat septa could be seen and differentiated from the surrounding tissue. The order was also checked to make sure that the sections were in the correct sequence. The images were then imported as a series into ‘Reconstruct’, a freely distributed 3D imaging software package obtain able from: http://synapses.clm.utexas.edu/tools/reconstruct/reconstruct.stm (Fiala, 2005). Structures of interest were traced manually in each image (optic nerve, globe, extra-ocular muscles, orbital walls, and fibrous septa) (Fig. 3). Once all the traces were complete, they were rendered into a 3D model by the imaging software.
The embedding procedure produced blocks of resin that were hard, transparent and with good optical qualities (Fig. 2). The sections that were produced were of good quality and able to be stained directly. However, it must be borne in mind that because of the thickness of the saw blade, 0.4 mm of tissue was lost with every cut.
Staining met with mixed success. The modified H & E stain was found to stain the septa only faintly, making it extremely difficult to pick out the fine septa when tracing the images (Fig. 4a).
The Lillie’s trichrome was found to be taken up by the resin, giving the whole section an intense yellow coloration that obscured the tissues of interest. There was also very little variation in soft tissue staining, with everything staining a uniform brown color (Fig. 4b). The modified Miller’s elastin stain was found to cause the resin to buckle and crack, as the embedded tissue absorbed alcohol and expanded. Furthermore the stain was found to have precipitated on the slide and collected in cracks in the resin, giving rise to numerous artefacts that obscured genuine staining (Fig. 4c).
With Gomori’s trichrome, it was found that the soft tissue structures were stained clearly and consistently (Fig. 5), although the intense red progressively faded with repeated use making the Celestine blue counterstain more obvious (Fig. 6). It is therefore recommended that a fresh batch of stain should be made up for each orbit. It was
also necessary to leave the slides in the stain for longer than the recommended period in order for the stain to be taken up fully by the section. However, leaving the slides immersed for over 4 hours can cause the tissue to expand and buckle, cracking and distorting the section. Gomori’s trichrome was found to stain collagenous structures well, but red-brown, rather than the expected green. This change in staining colour and tissue variation was evident in all the stains tested, suggesting that epoxy impregnation can affect the way tissues react to stains.
The images of the sections stained with Gomori’s trichrome proved adequate for tracing the soft tissue structures so that an accurate 3-D model of the retrobulbar septa was produced (Fig. 7). Other important soft-tissue structures of the orbital cavity were also traced and colored (Fig. 8). The rendered model can be rotated in 3D space as well as having structures removed and isolated. The septa that have been traced in each section can also be separated and rotated on their own.
E12 epoxy resin imbedding was found to be a very reliable method for creating orbit sections with excellent optical qualities. It was also found to create very little specimen distortion as the hardness of the block meant that structural relationships remained consistent throughout the sectioning process. Gomori’s trichrome proved to be an effective stain to highlight the septa and other soft-tissue structures within the orbit, enabling the structures of interest to be traced on the computer screen.
It was a notable finding of this study that embedding the specimens in epoxy resin instead of paraffin wax changed how the stains functioned. The protocols for histological staining are generally intended for wax-embedded microtome sections of 10 µ thickness or less. We found firstly, that staining times had to be increased to allow the stain to penetrate the relative thick section, and, secondly, that the colours of the different stained tissues were markedly different from those described in the protocols given for paraffin wax-embedded specimens. This variation seems to contradict the work of Johnson and Barnett (2005), in which the staining protocols for spinal soft tissues did not have to be altered for epoxy-embedded sections.
Due to limitations of time and materials, 3-D reconstruction could only be carried out on a single orbit, however, the resulting image clearly shows the complexities of the fibrous support network of the interlobular septa. The image can be rotated and manipulated in 3-D space, giving ophthalmic surgeons an unparalleled view of the area into which they operate. Future studies will compare images from different individuals to assess the degree of individual variation in the distribution pattern of the septa.
Furthermore, in this study, the septa and other soft tissue structures were traced manually with the mouse on the computer screen, which was a very time-consuming and painstaking process. For future investigations, the authors intend to investigate the use of imaging software that can automate the process by automatically selecting structures of the same color.
Limitations
This method of cutting semi-thin sections with a slow-speed diamond saw means that for every 0.3 mm-thick section that is cut, 0.4 mm will be lost due to the thickness of the blade. At best then, this method samples the specimen at 0.7 mm intervals. Another problem was that the tissues stained a different colour to what was expected, which meant that it was not possible to rely on the colour of the stain to identify the tissue.
In conclusion, E12 plastination combined with a modified Gomori’s trichrome stain provides a reliable method for visualising the connective tissue fat septa, as well as other soft tissue structures, of the orbit. This method could therefore be used with a greater number of orbits to find variations in the connective tissue architecture. It could also be used to ascertain whether these tissues make up a dynamic network which has a uniformity between individuals, providing vital support to the globe and communicating with the other structures within the orbital cavity to allow controlled locomotion.
Appendix
Gomori's trichrome stain:
distilled water --------------------- 200.0 ml
chromotrope 2R (CI 16570) ------- 1.2 g
light green SF (CI 42095) ----------- 0.6 g
dodecatungstophosphoric acid --- 1.6 g
glacial acetic acid ------------------- 2.0 ml
Dissolve each reagent separately in 50 ml aliquots of the distilled water, then mix all four solutions together. Allow to stand overnight, filter into a reagent bottle. The stain keeps well.
Adds PJ, Al-Rekabi A. 2014: 3-D reconstruction of the ethmoidal arteries of the medial orbital wall using Biodur® E12. J Plast 26: 5-10.
https://doi.org/10.56507/ELZV6849
Bremond-Gignac D, Copin H, Cussenot O, Lassau J-P, Henin D. 2004: Anatomical histological and mesoscopic study of the adipose tissue of the orbit. Surg Radiol Anat 26: 297-302.
https://doi.org/10.1007/s00276-004-0223-5
Fiala, JC. 2005: Reconstruct: a free editor for serial section microscopy. J Microscopy 218: 52-61
https://doi.org/10.1111/j.1365-2818.2005.01466.x
Gaudiani JL, Braverman JM, Mascolo M, Mehler PS. 2012: Ophthalmic changes in severe anorexia nervosa: a case series. Int J Eating Dis 45: 719-721.
https://doi.org/10.1002/eat.20970
Gomori, G. 1950: Aldehyde fuchsin: a new stain for elastic tissue. Am J Clin Path 20: 665
https://doi.org/10.1093/ajcp/20.7_ts.665
IHCWORLDa. Gomori's trichrome staining protocol for connective tissues. Available at: http://www.ihcworld.com/_protocols/special_stains/gomori's_trichrome_ellis.htm (Accessed: 7 March 2016).
IHCWORLDb. Hematoxylin and Eosin (HE) staining protocol. Available at: https://www.ihcworld.com/_protocols/special_stains/h&e_ellis.htm (Accessed: 7 March 2016).
IHCWORLDc. Miller's elastic staining protocol. Available at: http://www.ihcworld.com/_protocols/special_stains/miller's_elastic_ellis.htm (Accessed: 7 March 2016).
https://doi.org/10.17504/protocols.io.e23bggn
Johnson G, Barnett R. 2005: A comparison between epoxy resin slices and histology sections in the study of spinal connective tissue structure. J Int Soc Plastination 15: 10-13.
https://doi.org/10.56507/CXGV7781
Koornneef L. 1988: Eyelid and orbital fascial attachments and their clinical significance. Eye 2: 130-134.
https://doi.org/10.1038/eye.1988.26
Koornneef L, Zonneveld F. 1985: Orbital anatomy; the direct scanning of the orbit in three planes and their bearings on the treatment of motility disturbances of the eye after orbital "blow-out" fractures. Acta Morphol Neerl Scand 23:229-24.
Lillie RD. 1954: Histopathologic Technic and practical Histochemistry 3rd ed. United States: McGraw-Hill Inc.
Lillie's Trichrome for muscle and collagen. 2005: Available at: http://stainsfile.info/StainsFile/stain/conektv/tri_lillie.htm (Accessed: 8 February 2016).
Mawhinney WH, Richardson E, Malcolm AJ. 1984: Control of rapid nitric acid decalcification. J Clin Pathol 37: 1409-1413.
https://doi.org/10.1136/jcp.37.12.1409
Miller JM, Demer JL, Poukens V, Pavlovski DS, Nguyen HN, Rossi EA. 2003: Extraocular connective tissue architecture. J Vision 3: 240-251.
https://doi.org/10.1167/3.3.5
Miller P. 1971: An elastin stain. Med Lab Technol 28: 148-149.
Orbital connective tissue and fat. Available at: http://clinicalgate.com/the-orbit-and-accessory-visual-apparatus/ (accessed 7/9/2016)
Richter DF, Stoff A, Olivari N. 2007: Transpalpebral decompression of endocrine ophthalmopathy by intraorbital fat removal (Olivari technique): experience and progression after more than 3000 operations over 20 Years. Plast Reconstr Surg 120: 109-123.
https://doi.org/10.1097/01.prs.0000263655.47148.9e
Sora M-C, Strobl B, Staykov D, Förster-Streffleur S. 2004: Evaluation of the ankle syndesmosis: a plastination slices study. Clin Anat 17: 513-517.
https://doi.org/10.1002/ca.20019
Sora M-C. 2007: Epoxy plastination of biological tissue: E12 ultra-thin technique. J Int Soc Plast 22: 40-45.
https://doi.org/10.56507/TQMH6049