Anatomical Institute I The University of Heidelberg D-6900 Heidelberg West Germany
After distention of the joint capsule by formalin injection, a knee joint can be plastinated and used as a natural model for arthroscopy. Maintaining a distended capsule during the curing of the impregnation polymer provides a well- defined and permanently expanded joint cavity. The whole process, including a second defatting and impregnation (required for a completely acceptable specimen), takes 10 weeks. While not an exact model of natural structure, the plastinated knee joint specimen provides many important advantages in arthroscopy training.
Knee; Silicone; S10; Biodur
Klaus Tiedemann Anatomical Institute I The University of Heidelberg D-6900 Heidelberg West Germany
![]()



During conventional dissections of the knee joint, the boundaries and attachments of the synovial capsule remain indistinct. Specimens exhibiting a distended joint capsule (induced by either synovial effusion or injection) are particularly valuable for orthopedic or anatomic instruction because they enable the limits of this capsule to be more clearly demonstrated.
In the last decade, operative arthroscopy has become increasingly important (1)(2). The teaching of arthroscopy and arthroscopic surgery would benefit from the availability of specimens that would permit simulation of actual clinical procedures, particularly if such specimens were to incorporate a distended capsule. Knee joint specimens, plastinated with Biodur S10/S3 (3), make ideal, natural models of this kind. Used intact they permit rehearsal of arthroscopic procedures. Cut into halves, they reveal most anatomic details of the joint and suprapatellar bursa.
PLASTINATION OF A KNEE JOINT WITH DISTENDED CAPSULE
SPECIMEN SELECTION:
Only knee joint specimens from fresh bodies should be used. A joint capsule from an embalmed cadaver will not expand sufficiently. The entire specimen should be approximately 25 cm in length. Saw cuts should be made 10 cm below and 15 cm above the femorotibial joint.
FIXATION:
Fixation is begun with the injection of 20% formalin into the capsule, using a 12-15 gauge needle. When working without an arthroscope, the usual clinical approaches (anterolateral or anteromedial) are not recommended. The injection may over-infiltrate the infrapatellar fat pads
(4) and, two months later, the joint may become filled with a meringue-like mass. Superomedial or superolateral injection (into the suprapatellar bursa) is less precarious. The joint capsule is filled, close to bursting, with 120-250 ml of fixative. During injection, the patella must lift off from the trochlear groove and the knee will assume approximately 20 degrees of flexion. When the injection is complete, the joint is immersed in 5% formalin for about one week. The needle and syringe are left in place to maintain pressure within the capsule. Application of a gauze binding will help preserve the natural shape of the severed muscles.
DEHYDRATION:
Before dehydration, the specimen is rinsed with running water to remove most of the formalin. The dilated joint capsule is flushed several times, using the injection needle as a means of access. This same puncture site is used for all treatment of the joint capsule, including the final curing. Dehydration is accomplished by freeze-substitution with acetone. The joint is blotted, the capsule filled with acetone (precooled to -25°C) and the joint immersed in a 5x volume bath of this same agent. Dehydration will require three weeks. The acetone is maintained at -25°C in a freezer and the joint capsule is rinsed occasionally. Two changes of the acetone bath are mandatory. The joint is then transferred to a room-temperature solvent bath for defatting. This will require at least three days in each of two baths of either acetone or methylene chloride.
FORCED IMPREGNATION:
The specimen must now be impregnated with Biodur S10/S3 polymer mixture at -25°C. The first step is to remove the defatting solvent from the joint capsule and refill it with polymer. The entire specimen is then immersed in this same mixture. Vacuum impregnation is started at a negative pressure of 60 mm Hg during the first day and adjusted down to zero over a period of 12 days, at which time, impregnation should be complete.
CURING:
Before curing, the joint capsule must be emptied of all excess polymer. This is accomplished by introducing compressed air via the injection needle. Within two hours, the fluid silicone polymer should be completely forced out through the puncture site and the porous walls of the capsule. The joint capsule must now be cured, starting from the inside. This is done by blowing gaseous Biodur S6 curing agent into the joint space. A gas washing bottle is assembled in such a way that compressed air is led into a chamber containing 100 ml of S6 solution. Incoming air percolates through this solution, vaporizing the S6 and carrying it into the capsule (Figure 1). Gas flow is permitted to continue for one day under a fume hood. The needle is then withdrawn and the the whole specimen cured for 4 days according to the usual S6 procedure.
The specimen is now ready. After drilling a hole for the anterolateral or anteromedial approach, it can be used for teaching. At this stage, we prefer to cut the specimen into sagittal halves with a band saw. This permits direct inspection of the joint cavity. Also, the specimen can be reassembled and held intact by a gauze stocking' for
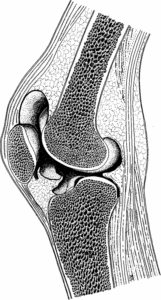
Figure 2 Plastinated knee joint with
expanded joint cavity, lateral compartment and gutter. This half of a natural model for arthroscopy shows the most instructive cutting plane. The cruciate ligaments are preserved in the other half.
simulated arthroscopy. The optimal plane of section runs through the middle of the femur, patella and tibia, parallel to the ligamentum mucosum (Figure 2).
We have found that fat remaining in the marrow cavity will become rancid after two months. Therefore, we recommend that the cured specimen halves be defatted once again for six days in two baths of pure, room-temperature acetone, re-impregnated with S10/S3 and re-cured in the usual manner.
The technique outlined in this paper for pressure distention of the capsule of the knee joint can be used for all joints of relevance to arthroscopy. Today, even small joints are inspected with the arthroscope (5). The elbow joint, for example, may be examined, almost in its entirety, by using a posterior radial approach. Even the posterolateral pouch of the ankle joint can be seen with a posterolateral approach. Similarly, bronchial trees, that can serve as natural models for bronchoscopy, can be obtained, by preparing lung/airway specimens in a comparable manner.
The actual operative effort required for completing a knee joint specimen as described here is not excessive, however, because of the sometimes-lengthy segments of time needed for soaking and impregnation, at least 10 weeks are required before the specimen can be used. This includes the second defatting and impregnation which is recommended because it precludes offensive odor and renders the specimen less greasy to the touch.
The two most common pitfalls in the procedure are insufficient expansion of the capsule or, at the other extreme, rupture of the capsule (usually into the gastrocnemius or suprapatellar bursa). According to clinicians, it takes between 60 and 100 ml to fill the cavity (4). Some authors advocate the use of an infusion bottle, hung just below the ceiling, rather than syringe pressure, for distention (2).
During subcutaneous dissection, piercing of the fasciae must be avoided. All-attempts to cut away muscles also will endanger the integrity of the capsule and may permit its communication with numerous synovial bursae about the knee. With radiography, especially CAT scans, both expansion of the capsule and the plane of the final sagittal section can be monitored.
Although a knee specimen, as prepared here, is very close to natural, it cannot provide all of the desirable characteristics of a fresh joint. Normal flexion and extension is not possible because the fully cured silicone rubber is not sufficiently supple. Application of varus and valgus stress (to expose the posterior meniscus) also is not feasible. Despite these limitations however, actual comparison has shown that a well-expanded, plastinated knee exhibits more than 80% of the details that can be studied arthroscopically in the patient. Also, plastinated structures look enough like their fresh counterparts to generate familiarity with their natural appearance. Many objections can be overcome by having a second joint, plastinated in a more flexed position, to complement the first one.
On the other hand, a specimen of this type provides certain unique advantages. For example, when the patella stands 3mm away from its articular surface on the femur (as it does in these preparations), the suprapatellar bursa and the cartilagenous face of the patella both can be inspected through an insertion hole aimed primarily at the cruciate ligaments and the anterior horns of the menisci (anterolateral approach).
For training of the beginner in arthroscopy, the ability to study the cut surface of the joint is an important asset. In fact, it has been our experience that every orthopedist who was given the opportunity to examine a distended, plastinated knee joint wanted to obtain one for his own use.
1. Guhl, JF Evolution and development of operative arthroscopy: 1974 to present. Orthopedics 6:1104, 1983.
https://doi.org/10.3928/0147-7447-19830901-04
2. Glinz, W Diagnostische Arthroskopie und arthroskopische Operationen am Kniegelenk. Huber, Bern, 1987.
3. Hagens, Gv ; Tiedemann, K ; Kriz, W The current potential of Anat Embryol 175:411-421, 1987
https://doi.org/10.1007/BF00309677
4. O'Connor, RL ; Shahriaree, H Arthroscopic techniques and normal anatomy of the In: O'Connor's textbook of arthroscopic surgery, H. Shahriaree (Ed.) Lippincott, Philadelphia, pp 43-71, 1984
5. Watanabe, M Arthroscopy of small Igaku-Shoin, Tokyo - New York, 1985