Department of Comparative Medicine, College of Veterinary Medicine, University of Tennessee, 2407 River Drive, Knoxville, TN, 37996, USA.
To determine the involvement of acetone in the discoloration of cured epoxy polymer, epoxy reaction-mixture was combined with different concentrations of acetone before being allowed to cure at either one atmosphere or full vacuum. Acetone was found to have a direct impact on the degree of discoloration of cured epoxy reaction-mixture. Curing the epoxy reaction- mixture under vacuum decreased discoloration caused by the acetone.
plastination; epoxy; acetone; discoloration
![]()



Tissue slices plastinated with epoxy polymer are finding their way into medical research (Guhr et al., 1987; Phillips et al., 2002) and education (Cook, 1997a; Cook 1997b). Being used in these disciplines, it is necessary to minimize artifacts introduced during and after the plastination procedure to ensure accurate assessment of tissue samples. Attempts have been made to increase the transparency of specimens plastinated with epoxy polymer (Mathura and Satyapal, 2000) as well as to decrease the yellowing of cured epoxy following plastination (Latorre et al., 2002). Improved techniques for epoxy plastination have been reported but do not include methods for reducing or eliminating the discoloration of cured epoxy (Fasel et al., 1988; Alston et al., 1997). Shrinkage of tissues plastinated via the E12 technique has been measured and documented (Sora et al., 2002) yet no information is currently available documenting the discoloration of cured epoxy polymer.
Acetone has classically been used as the dehydrating agent for epoxy plastination (von Hagens, 1989; Weber and Henry, 1993; Sora and Cook, 2007; Cook, 2007). Experiments have been performed to determine the optimum method for using acetone as a dehydrating agent for tissue (Holladay, 1988; Brown et al., 2002). However, investigations are lacking in the area of determining possible unwanted side effects of the acetone dehydration on different plastination procedures. This experiment was designed to determine if acetone is involved in the yellow discoloration evident in cured epoxy polymer.
E1 and E12 were mixed at 95 parts of polymer to 26 parts of hardener by weight as per von Hagens (1989). Glass separator (AE30) was intentionally omitted from the reaction-mixture to eliminate any involvement it may have in the discoloration of epoxy polymer. Forty milliliters of this epoxy reaction-mixture was poured into each of fifteen 50 ml glass beakers. The beakers were randomly divided into five groups of three beakers each. The beakers in group 1 were used as experimental controls. One milliliter of acetone was added to each of the beakers in groups 2 and 3 and stirred into the epoxy reaction-mixture. Eight milliliters of acetone was added to each of the beakers in groups 4 and 5 and stirred into the epoxy reaction-mixture. The beakers in groups 1, 3 and 5 were left uncovered on a counter top for 24 hours.
The beakers in groups 2 and 4 were placed into a vacuum chamber. The pressure in the vacuum chamber was slowly decreased over six hours to the point of full vacuum. The beakers remained at the point of full vacuum for 24 hours. The epoxy reaction-mixture in the beakers of all groups was examined for curing after 24 hours.
The hardened epoxy reaction-mixture in all groups was stored in the dark and evaluated for signs of discoloration at 24 hours, six months and twelve months post experimentation.
The epoxy contents of all beakers had cured after 24 hours. All reaction-mixtures appeared clear 24 hours following curing regardless of the exposure or non- exposure to acetone or vacuum. The control beakers containing epoxy reaction-mixture in the absence of acetone remained clear six months after curing (Figs. 1 and 2).

Figure 1. Epoxy reaction-mixture cured for 6 months. Reaction-mixture control (A), reaction-mixture with 1.0ml acetone and subjected to vacuum (B), reaction-mixture with 1.0ml acetone (C), reaction-mixture with 8.0ml acetone and subjected to vacuum (D), reaction-mixture with 8.0ml acetone (E).
At six months post-experimentation, all beakers containing the reaction-mixture and 1.0ml of acetone, regardless of whether they were placed under vacuum or not, showed only a very slight yellowing that was best observed when viewing the beaker from the side (Fig. 1). The cured reaction-mixture exhibited a slightly darker yellow discoloration at its surface (Fig. 1). This greater discoloration extended 2.0mm into the cured polymer and was not visible when viewing the cured epoxy from above (Fig. 2).
The beakers containing epoxy reaction-mixture and 8.0 ml of acetone that were exposed to vacuum showed a yellowing of the cured mixture that was greater than that evident in beakers treated with 1.0ml of acetone (Figs. 1 and 2).

Figure 2. Epoxy reaction-mixture cured for 6 months (same samples as those in figure 1). Reaction-mixture control (A), reaction-mixture with 1.0ml acetone and subjected to vacuum (B), reaction-mixture with 1.0ml acetone (C), reaction-mixture with 8.0ml acetone and subjected to vacuum (D), reaction-mixture with 8.0ml acetone (E).
These beakers also exhibited yellowing at the surface at six months post-experimentation to a greater degree than did the beakers containing 1.0ml of acetone (Fig. 1). The yellowing was first evident at four months post-experimentation. This yellow discoloration extended 6.0 - 8.0mm into the cured polymer. The yellow discoloration of the cured epoxy reaction- mixture was evident in these beakers when viewing them from above (Fig. 2).
The beakers containing epoxy reaction-mixture and 8.0mls of acetone and allowed to cure at 1.0 atmosphere exhibited a marked yellow discoloration throughout the extent of the cured epoxy at six months post- experimentation when compared to all other test mixtures. (Figs. 1 and 2). This discoloration first became evident 3 months post-experimentation.
Between six months and one year post- experimentation, the glass beakers began to crack and subsequently fall away from the cured reaction-mixture. At one year post-experimentation, control beakers and all beakers containing 1.0ml of acetone showed a very light yellowing throughout the cured

Figure 3. Epoxy reaction-mixture cured for 1 year (same samples as those in figure 1). Reaction-mixture control (A), reaction- mixture with 1.0ml acetone and subjected to vacuum (B), reaction-mixture with 1.0ml acetone (C), reaction-mixture with 8.0ml acetone and subjected to vacuum (D), reaction-mixture with 8.0ml acetone (E).
reaction- mixture (Figs. 3 and 4).
The yellowing was slightly less evident in the control beakers when viewed from the side (Fig. 3). The beakers containing the reaction- mixture with 8.0mls of acetone that were placed under vacuum exhibited a deep yellowing of the cured reaction-mixture (Figs. 3 and 4). Beakers containing the reaction-mixture with 8.0mls of acetone that were not subjected to vacuum showed a marked discoloration of the cured reaction-mixture to the point it appeared orange (Figs. 3 and 4).
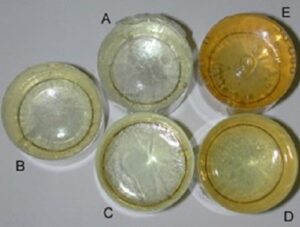
Figure 4. Epoxy reaction-mixture cured for 1 year (same samples as those in figure 1). Reaction-mixture control (A), reaction- mixture with 1.0ml acetone and subjected to vacuum (B), reaction-mixture with 1.0ml acetone (C), reaction-mixture with 8.0ml acetone and subjected to vacuum (D), reaction-mixture with 8.0ml acetone (E).
The epoxy reaction-mixture control samples remained clear longer and yellowed to a lesser degree than all the others due to the lack of effects of acetone on the cured mixture. The fact that it did discolor to some degree suggests that acetone is not required to be present for epoxy reaction-mixture to discolor in some manner.
The reaction-mixtures to which 1.0ml of acetone was added cured no differently from one another, whether or not vacuum was applied. When these were compared to the control beakers, they did discolor to a greater degree which can only be attributed to the presence of the acetone. The fact that there was no discernable difference between those beakers to which vacuum was applied and those which were not subjected to vacuum would suggest that the mere presence of 1.0ml of acetone was enough to discolor the surface of the cured reaction-mixture at six months and discolor the entire mixture at one year whether the acetone was forcibly removed or not. As there was a difference between beakers containing 8.0mls of acetone when subjected to vacuum compared to those that cured at 1.0 atmosphere, we may conclude that the vacuum was able to remove some acetone from the reaction-mixture. In comparing the beakers to which 1.0ml of acetone was added to those with 8.0mls, it appears that vacuum is able to remove acetone to some degree yet not entirely. It cannot be determined from these experiments if the vacuum was unable to remove all of the acetone prior to the acetone affecting the reaction-mixture or if vacuum simply cannot remove all acetone before the reaction-mixture cures and that which is left will then act to discolor the reaction- mixture. Additionally, is vacuum unable to extract acetone once acetone decreases to an undetermined concentration in the reaction-mixture.
The reaction-mixtures to which 8.0mls of acetone were added were markedly discolored by the presence of the acetone regardless of the effects of the vacuum. The results using 8.0mls of acetone clearly demonstrate that if acetone can be removed from the reaction- mixture before it cures, specimens will exhibit less discoloration after the passage of time.
Epoxy reaction-mixture used for impregnation is regularly used for casting the impregnated tissue. As soon as tissue ceases to release acetone bubbles, impregnation is considered complete, however, the reaction-mixture will be saturated with acetone. It would stand to reason that the sooner the impregnation reaction-mixture is used for casting following the completion of impregnation, the greater the probability for epoxy yellowing following curing. In actuality, the time that exists between completion of impregnation and curing of the reaction-mixture is most likely too short for much acetone to escape through the surface of an ever hardening mixture. This technique will encapsulate the tissue slice with reaction-mixture containing acetone which will eventually lead to discoloration of the specimen. If cost is not an issue in specimen preparation, it would be best to use freshly mixed epoxy reaction-mixture which has never been in contact with acetone to plate the tissue slices. This should keep discoloration to a minimum. If a vacuum is applied to reaction-mixture which has been used for impregnation before it is used for casting, it should eliminate some acetone. This application of vacuum will also cause the release of bubbles from the reaction- mixture which are not related to acetone and which should not be confused for a highly acetone saturated reaction-mixture as this bubbling will not cease until the curing reaction-mixture becomes so viscous that bubbles can no longer escape (Reed, 2003).
The cured epoxy reaction-mixtures shown in figures 3 and 4 are not contained within a glass beaker. Over time, the contracting reaction-mixture pulled on the glass and caused the glass beakers to crack and subsequently fall away as there was no glass separator included in the experiment.
Alston M, Janick L, Wade RS, Weber W, Henry RW. 1997: A shark band saw blade enhances the quality of cut in preparation of specimens for plastination. J Int Soc Plastination 12(1):23-26.
https://doi.org/10.56507/OLQF4216
Brown MA, Reed RB, Henry RW. 2002: Effects of dehydration mediums and temperature on total dehydration time and tissue shrinkage. J Int Soc Plastination 17:28-33.
https://doi.org/10.56507/XNQM4606
Cook P. 1997a: Sheet plastination as a clinically based teaching aid at the University of Aukland. Acta Anat 158(1):33-36.
https://doi.org/10.1159/000147907
Cook P. 1997b: Submacroscopic interpretation of human sectional anatomy using plastinated E12 sections. J Int Soc Plastination 12(2):17-27.
https://doi.org/10.56507/XICY2283
Fasel J, Mohler R, Lehmann B. 1988: A technical note for improvement of the E12 technique. J Int Soc Plastination 2(1):4-7.
https://doi.org/10.56507/LNBR6798
Guhr A, Mueller A, Anton H, von Hagens G. 1987: Complete examination of mastectomy specimens using sheet plastination with epoxy resin. J Int Soc Plastination 1(1):23-29.
https://doi.org/10.56507/NXYR1705
Holladay SD. 1988: Experiments in dehydration technique. J Int Soc Plastination 2(2):17-20.
https://doi.org/10.56507/RSDZ6598
Latorre RM, Reed RB, Gil F, Lopez-Albors O, Ayala MD, Martinez-Gomariz F, Henry RW. 2002: Epoxy impregnation without hardener: to decrease yellowing, to delay casting and to aid bubble removal. J Int Soc Plastination 17:17-22.
https://doi.org/10.56507/LZKY8224
Mathura G, Satyapal KS. 2000: Quest for transparency in plastination. J Int Soc Plastination 15(1):14-17.
https://doi.org/10.56507/FQME4545
Phillips MN, Nash LG, Barnett R, Nicholson H, Zhang M. 2002: The use of confocal microscopy for the examination of E12 sheet plastinated human tissue. J Int Soc Plastination 17:12-16.
https://doi.org/10.56507/LPFJ4438
Reed R.B. 2003: Effects of reduced pressure on components of epoxy (E12) reaction mixture. J Int Soc Plastination 18:3-8.
https://doi.org/10.56507/XKIZ4979
Sora MC, Brugger PC, Strobl B. 2002: Shrinkage during E12 Plastination. J Int Soc Plastination 17:23- 27.
https://doi.org/10.56507/DIUH4490
Sora M.C., Cook P. 2007: Epoxy plastination of biological tissue: E12 technique. J Int Soc Plastination 22:31-39.
https://doi.org/10.56507/FCTY3173
Sora M.C. 2007: Epoxy plastination of biological tissue: E12 ultra-thin technique. J Int Soc Plastination 22:40-45.
https://doi.org/10.56507/TQMH6049
von Hagens G. 1989: BiodurTM Products: Polymers, auxiliaries and equipment for plastination. A catalog and price list. Rathausstrasse 18, Heidelberg, Germany: Biodur, p. 34-35.
Weber W, Henry RW. 1993: Sheet plastination of body slices - E12 technique, filling method. J Int Soc Plastination 7:16-22.
https://doi.org/10.56507/EZGX2343