Department of Pharmacology, University of Lund, Lund, Sweden
Use of acetone as a common volatile intermediary for the plastination process entails problems in containing its flammable vapors. During the freeze-substitution stage of the plastination process, acetone vapors can be contained by using multiple heat sealed polyethylene bags containing heavy duty polyethylene boxes in which the specimens are placed.
Dehydration, Equipment, Safety
Michael T. E. Fahlman, Department of Pharmacology, University of Lund, Solvegatan 10, S-223 62 Lund, Sweden. Telephone: 46 46 222 72 39 / Fax: 46 46 222 44 29. Email: Michael.Fahlman@MPHY.LU.SE
![]()



The plastination process requires the replacement of tissue water with a miscible organic solvent acting as a vola- tile intermediary for the plastination compounds. One such common solvent is acetone (von Hagens, 1985; Henry, 1991). However, acetone is considered highly flammable and explosive in its vapor state. Attempts to set up a plastination laboratory in the department of Anatomy, Lund, Sweden, were stopped by the faculty due to the acetone vapor problem. A method for storing and handling acetone and for freeze-substituting with acetone with a minimum of acetone vapor escaping into freezers and the laboratory atmosphere is described.
Acetone (99%) was purchased in soft plastic one-liter bottles from a local paint and chemical store. The bottles deformability reduced the risk of accidents which can occur with the standard glass bottles when stored in the cold or handled when cold.
Three-liter capacity heavy duty polyethylene freezer bags (not the zip-lock type, though these may serve well too) were purchased from a local grocery store, as were 3 two-liter high density polyethylene ice cream boxes. The plastic bags were tested for leaks by either filling with water, or by blowing them up by mouth and squeezing the air-filled bag. Leaking bags were discarded.
Specimens chosen were: a small intestine with mesentery (volume: 850 cc); a colon (volume: 700 cc) and a stomach together with a caecum-ascending colon specimen (combined volume: 250 cc).
Each acetone bottle was placed in a plastic bag, excess air was gently squeezed out of the bag, and the open end of the bag heat sealed with an Impulse Sealer (TEW(QS), type TISH(QS)300, O. Mollerstrom AB, Gothenburg, Sweden) set at 50%, for 2 seconds. Each bagged acetone bottle was then placed in a second plastic bag which was also heat sealed. The process was repeated a third time, for a total of 3 bags enveloping each bottle. All work done with acetone and the Impulse Sealer was done in a draft cupboard. The acetone bottles were then stored in a freezer chest at -25°C. for 1 month. The freezer and heat seals were checked for acetone vapor leaks on a weekly basis. As no leakage could be detected, work with specimens was initiated.
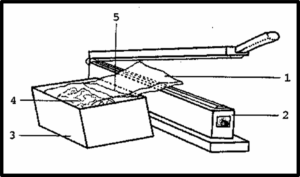
Figure 1. Heat-sealing of the specimen bag. 1 - specimen bag, 2 - heat sealer, 3 - polyethylene specimen box (note tilting of box away from heat sealer, preventing acetone spillage), 4 - specimen in bag, 5 - acetone level in bag. (figure by the author)
The 3 specimen groups were each placed in a plastic freezer bag, each of which was placed in a polyethylene box. A bottle of the -25°C. cooled acetone was emptied into each specimen bag. The top of the specimen bag was adjusted so it hung over the edge of the box. The opposite side of the box was held slightly lower while air in the bag was gently pressed out, taking care not to get any acetone over the edge of the box! The protruding part of the bag was heat sealed promptly (figure 1). The heat sealed specimen bag was then completely tucked inside the box and the box's lid pressed down in place. The box was then placed in a plastic bag, which was also heat sealed. This was in turn placed in an outer plastic bag, which was also heat sealed. All three specimen boxes were processed in the same manner and stored in the same freezer chest as the acetone bottles. The freezer and outer plastic bag's heat seals were checked once every week for acetone vapors.
Acetone was changed every third week. The outer plastic bags surrounding each specimen box were cut open at the heat seal. A funnel was inserted into the neck of an empty acetone bottle, a 5 cm hole was cut below the heat seal of the specimen bag and the waste acetone drained into the bottle. The specimen was then swiftly transferred to a new bag and placed in its box. To minimize acetone evaporation, the old acetone soaked specimen bag was immediately placed in a plastic freezer bag which was subsequently placed in a plastic trash bag whose neck was twisted tight after each acetone change. Next, a bottle of acetone pre- cooled to -25°C was added to each specimen bag, which was then heat sealed as described above. Each specimen box was finally placed in double heat sealed bags as described above and stored in the freezer chest. In all, 4 changes of acetone were made on each of the 3 specimen boxes. More changes were deemed unnecessary, as a tissue volume to acetone ratio of 1:5 has been shown to be adequate for dehydration (Tiedemann and Ivic-Matijas, 1988).
The water content of the waste acetone could not be measured since such instrumentation was unavailable.
A total of 12 acetone changes were made during the course of the experiment. No acetone vapors were detect- able in the freezer. A 1 week period should give a leak adequate time to make itself noticeable. No acetone vapor leakage was detectable from the outer bags surrounding the polyethylene containers.
Pre-cooling acetone to -25°C, which is below acetone's flash point of -18°C (Budavari S, 1996), minimizes shrink- age of tissues (von Hagens, 1985). Pre-cooling thus also reduces acetone evaporation during acetone changes, which is important since the heat sealer uses electricity. During acetone changes, the lower evaporation rate of cold acetone was taken advantage of to minimize vapor formation by swiftly transferring the used specimen bags to a fresh plastic bag for disposal.
At each acetone change, the inside of each polyethylene box was checked for acetone leakage. In 2 cases acetone had leaked out of the specimen bag into the box. Careful inspection of the heat seals of the leaking bags showed that one had not sealed properly due to doubled up wrinkles at one point across the seal. Wrinkles crossing the line of sealing were carefully avoided thereafter. The other leaking specimen bag had a half-mm hole in one end of the seal where the plastic had melted excessively during sealing. However, the outer bags surrounding the polyethylene boxes had successfully prevented acetone vapors from escaping into the freezer, which was the principal reason for devising this method of freeze-substitution.
Unfortunately, the experiment could not be brought to its conclusion, ie- S-28 plastinated specimens, due to the closure of the anatomy department with cessation of all work with human specimens.
This method, if meticulously applied, should permit freeze substitution in any freezer without risking acetone vapor escape, fire or explosion. For larger specimens, cus- tom sized bags could be made from heavy gauge polyethylene sheeting with an appropriate heat sealing ap- paratus designed for such purpose. However, an adequate draft cupboard for any specimen size and acetone quantity is a must when changing or otherwise working with acetone in the open.
Budavari S: The Merck Index, 12th Ed. Whitehouse Sta- tion, NJ: Merck & Co., P 59,1996.
Henry RW: S/10 Plastination Techniques. Second Interim Conference on Plastination Workbook: 9-17, 1991.
Tiedemann K, Ivic-Matijas D: Dehydration of Macroscopic Specimens by Freeze Substitution in Acetone. J Int Soc Plastination 2 (2): 2-12, 1988.
https://doi.org/10.56507/SCLL2742
von Hagens G: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomisches Institut 1, Universitat Heidelberg, Heidelberg, Germany, 1985.