1Department of Anatomy, Medical faculty, Tracian University, St. Zagora, Bulgaria
²Department of Ophthalmology, Medical faculty, Tracian University, St. Zagora, Bulgaria
³Department of Ophthalmology, Medical University, Plovdiv, Bulgaria
Pig eyes were prepared, using a combination of Silicone S10 infiltration and polyethylene glycol impregnation. The vitreous body (corpus vitreum) was liquified, withdrawn and replaced by silicone S10. The wall of the eyeball (bulbus oculi) was then impregnated with polyethylene glycol. This first attempt to combine two chemically-different polymers for the plastination of teaching specimens success- fully resulted in soft and life-like eyes. The specimens plastinated with this technique are safe for student handling and use during anatomical study. They are also unique for practicing delicate surgery procedures.
Ophthalmology, Polymer-SlO, Polyethylene glycol, Surgery, Teaching
Dr Dimiter Sivrev, Department of Anatomy, Histology and Embryology, Medical University, Armejska Str. 11, Stara Zagora 6000, Bulgaria. Telephone: 359 42 2819 265 / Fax: 359 42 331 98. Email: vmisz@bgcict.acad.bg
![]()



It is not always possible for the ophthalmology students' and post-graduate students' education to take place in real clinical settings (with live patients) because of the danger of injuring or even loosing eyesight as a result of the trainees' unskillful manipulations. Practicing on dead human or animal material is more complicated because of the irreversible post death changes in the organic components which occur under the influence of microorganisms and proteolytic enzymes contained within the tissues. Thus there is a need for developing improved techniques for preparing eye specimens suitable for training of ophthalmology students.
After several centuries of more or less successful at- tempts to develop techniques for preserving the organic matter (reviewed by Kurz, 1993) the plastination technique (von Hagens et al., 1987) has been widely acclaimed for preparing anatomically-accurate teaching specimens from human or animal tissues, and represents a major step for- ward over previous techniques. This technique involves solvent dehydration and subsequent impregnation of the actual animal tissue to be studied or manipulated. It largely preserves the anatomic integrity of the tissue. Polymers selected for the impregnation vary according to desired physical characteristics of the final product (Sivrev and Kayriakov, 1995), and commonly include methyl methacrylate, polyethylene glycol, epoxy resins and silicone derivatives (Whalley, 1994). This is the first report in which two chemically-different polymers have been successfully used to plastinate tissue for teaching purposes.
Pig eyes (which are close in size and structure to hu- man eyes) were selected for model development. Fixation was performed by immersion at room temperature.The fixa- tive solution used (Formaldehyde 10%, Ethanol 40%, Wa- ter 50%) (von Hagens, personal communication) preserved the specimen colour relatively similar to the natural one. The specimens were stored in fixative solution until ready for plastination. They can remain in this solution for unlimited time. The experiments carried out with 5% formaldehyde solution; Kaiserling solution (Kaiserling, 1896); and the Plasdoform based embalming fluid (Dodge Chemical Co., Cambridge, Massachusetts, USA) led to greater darkening of the specimen.
Following fixation, eyes were washed in running tap water. Blood traces remaining on them were then removed by the use of 0.5 - 3% solution of hydrogen peroxide. This procedure takes a few minutes. The whole process was con- trolled visually and discontinued immediately after the dis- appearance of the blood traces, so that a change in the tissue colour was avoided (lower concentration of hydrogen peroxide and longer duration of exposition produced better results). Afterwards, the specimens were once again washed in tap water. They were ready to be cleaned of excess tis- sues by dissection to levels suitable for intended practice surgery procedures.
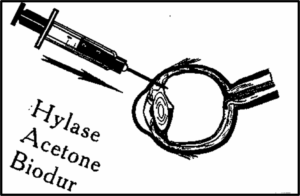
Figure 1. Steps of liquification and removal of the vit- reous body with Hylase; dehydration with acetone and in- filtration with silicone S10 are all performed by injection through the pars plana.
Hylase (Farma Dessau, Gbm, Germany) 300 UI was injected through the pars plana into the vitreous chambers to help liquification and removal of the vitreous body (figure 1). The specimens were incubated at 37°C for 2 hours. Dehydration was then performed using pure acetone (von Hagens, 1985). At this time, acetone was gently and repeatedly injected into, and then withdrawn from the vitreous chambers of eyes using a syringe and a broad lumen needle. Following several minutes of this procedure the acetone withdrawn from the vitreous chambers became clear, indicating that the hylase-digested vitreous body had been removed. If successful liquification had been achieved, only 3 changes of acetone were generally sufficient. Excess ac- etone was then drained from the vitreous chambers, after which Biodur S10/S3/S6 (100:1:0.7) silicone (von Hagens, 1987 ) was injected into the chambers until they were full. Specimens were then placed in the polyethylene glycol 400 (PEG 400) and stored at 5°C to permit the curing of the silicone.
The silicone filled specimens were afterward impregnated at room temperature under vacuum with the solution of PEG 400 (Steinmann, 1986) which impregnated the wall of the eyeball and associated structures (figure 2). The process lasted approximately 12 hours at a constant pressure of 11.5 to 19 mm Hg inside the impregnation chamber. The disappearance of the bubbles indicated the end of the impregnation process. After complete impregnation, the specimens were removed from the vacuum chamber and dried for several weeks by placing them on filter paper in a room protected from direct sun light (figure 3).
After drying, the specimens were soft, life-like and suit- able to be used in the educational process. In addition, the reversibility of the PEG impregnation process permits the correction of the specimens that were not properly impregnated. In these cases, there appeared shrinking of the specimens. The eyes were then completely immersed in water at room temperature. PEG dissolved and the entire impregnation process was repeated until we achieved satisfactory specimens (e.g. re-impregnation was performed). The appearance of the so-plastinated specimens is similar to their natural condition. In spite of a light darkening of the lens, these specimens proved to be excellent models for the ophthalmology-surgical educational process. Students and post-graduate students in ophthalmology may practice operating techniques on these specimens until the techniques are mastered, without any danger of eyesight loss due to any mistakes by the trainees as could happen with living patients.
The vitreous body is constructed of interlaced filaments, and the space between them is filled with liquid consisting of 99% water and 1% hyaluronic acid, as well as hydrophilic polysaccharides. This colourless and amorphous mucilagi- nous mass was replaced by Biodur S10. The cured silicone protects internal structures, maintains the shape of the eye and prevents the retina from detaching when the eye is placed under vacuum during the subsequent phases of the impregnation process. This makes the pig eye surgery models more representative of living human eyes. Eyes plastinated by this new technique are safe for student handling and use. They were prepared with a relative ease and limited expense, and are suitable for storage at room temperature in boxes lined with filter paper, rather than requiring submersion in formaldehyde-containing fixatives in glass jars. We found the combination of the two chemically different polymers (Silicone S10 and Polyethylene Glycol 400) an excellent method for the preparation of this new type of specimens. Eyes prepared by this new technique appear to represent improved specimens for teaching certain surgery procedures to ophthalmology students as compared to previously used eyes in our laboratory. Mastering of complicated techniques in the eye operations have been greatly improved in our laboratories by the use of these exceptional specimens. We are at the present time experimenting on the removal of the vitreous body by using the "pars plana-vitrectomy" method (Majdrakova et al., 1994). We expect it to be a more efficient method for the extraction of the vitreous body.
Kaiserling C: Ueber die Konservierung von Sammlung - spräparaten mit Erhaltung der naturlischen Farben. Klin. Wschr., Berlin 33: 775, 1896.
Kurz H: Entwigklung der modern Konservierung anatomischer Praparate. Basel, 1993.
Majdrakova IP, Chilova B, Balabanov Ch, Pencheva D, Mitov T, Philipov E: Ophthalmology. Publishing House "Kastalia", Plovdiv, 202 - 260, 1994.
Sivrev D, Kayriakov J: Plastination as an option for Anatomy Museum's collection. Xll Congress of Bulgarian Anato- mists with International Participation, Abstracts, p. 127, Plovdiv, 1995.
Steinmann W: Die Polyethilenglykol-Impregnation. Wienn, 1986.
von Hagens G, Tiedmann K, Kriz W: The current potential of plastination. AnatEmbryol 175: 411-421, 1987.
https://doi.org/10.1007/BF00309677
von Hagens G: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomische Institut 1, Universitat Heidelberg, Heidelberg, Germany, 1985.
Whalley A: Biodur TM products - Polymers, Auxiliaries, and Equipment for Plastination. Heidelberg, Germany, 1994.