1International Morphological Centre, Repishheva 9-94, 197375, Saint Petersburg, Russia
2 College of Veterinary Medicine, Lincoln Memorial University, Harrogate, TN, USA
A variety of organs, body regions and whole body specimens were plastinated using standard procedures for both cold and room temperature silicone plastination techniques. From these plastinates, advantages and shortcomings of both methods were evaluated. Criteria used for evaluation of plastinates included: duration of impregnation and curing, quality of plastinated specimens, need for extra equipment and its maintenance, as well as other cost considerations. To efficiently evaluate shrinkage and plastination duration, 3 cm pieces (core samples) of parenchymatous organs and 7 cm lengths from intestinal segments were collected, dehydrated and plastinated using standard procedures for both cold and room temperature silicone plastination techniques. Core sample volume was evaluated at the end of each stage of the process by fluid displacement. Shrinkage of samples was calculated after each stage of plastination. Evaluation of this information showed that the room temperature plastination technique takes about 35% less time for impregnation and curing, causes an average 8% less specimen shrinkage, produces life-like hair, fur or feathered specimens and it is more cost-efficient. The cold temperature plastination technique produces more flexible and elastic specimens and is preferable for whole body plastination.
cold technique; room temperature technique; shrinkage; silicone plastination
D. Starchik, telephone: 7-812-9569765; Fax: 7-812-3031853; E-mail: starchik@mail.ru
![]()



Introduction
The S10 plastination technique, using a cold impregnation reaction-mixture, was introduced by Gunther von Hagens in 1977 and now is the classic plastination technique which has been used worldwide (von Hagens, 1979a, 1979b, 1980, 1981, 1985; von Hagens et al., 1987; Bickley et al., 1981, 1987; Henry, 1987, 1995, 1998, 2007a; Weiglein and Henry, 1993; de Jong and Henry, 2007). The working material of this method is a silicone impregnation reaction-mixture, consisting of 99% polydimethylsiloxane with a relative molecular weight of 27200, plus 1% dibutyltindilaurate S3 as a catalyst (Chaynes and Mingotaud, 2004). This reaction-mixture becomes viscous when it is used or stored for several months at room temperature. To prolong the less viscous state needed for impregnation, impregnation is carried out in a deep-freezer; and when not in use, the polymer reaction-mixture is stored below -20°С.
In 1998, Daniel Corcoran, Dow Corning Corporation, proposed another sequence for combination of components for the silicone impregnation-mixture, and the room temperature plastination technique was developed (Glover et al., 1998; Henry et al., 2001; Latorre et al., 2001; Raoof, 2001; Glover, 2004; Henry, 2007b; Raoof et al., 2007). A non-reactive silicone of 92% low-molecular-weight polydimethylsiloxane and 5-8% of cross-linker CR-20 were used. This combination of silicone and cross-linker is stable at room temperature, therefore there is no need to impregnate specimens in a freezer. This is in contrast to the silicone-catalyst mixture, which is not stable at room temperature and needs to be used and stored in a deep freezer. Today about 80% of plastination laboratories in the world use the classical cold temperature technique; in spite of the ease of penetration of silicone and no need for additional equipment for the room temperature plastination technique, only 20% of all laboratories use this new plastination method (Starchik, 2014).
We assumed there were other quantitative and qualitative differences between cold and room temperature silicone plastination techniques besides polymer components and sequence of their combination. The purpose of this research was to compare methodologies and results of both standard methods and report the findings.
The following criteria were used to compare the two common silicone plastination techniques (cold vs room temperature) and two types of specimens (a. Normal plastinates [organs, regions of body and whole bodies] and b. Tissue core samples):
Specimens were collected and fixed in 10% formalin. To standardize experimental conditions two types of specimens were used:
First set of experiments (cold temperature (CT) protocol)
Impregnation was carried out with silicone mixture consisting of 99% S10 (relative molecular weight of 23000 to 24000; content of hydroxyl groups 0.57 - 0.60 %; kinematic viscosity at 20°C 360 - 400 cSt), and 1% catalytic agent S3 (dibutyltindilaurate) at -20°С. Pressure in the vacuum chamber was lowered slowly from 200 to 3 mm Hg maintaining a moderate boil. After impregnation was completed and excess polymer-mix drained, the specimens were placed for curing into a gas curing chamber with cross-linker S6 (tetraethoxysilane) vaporized by an aquarium pump, for 10 - 12 hours (von Hagens, 1987).
Second set of experiments (room temperature (RT) protocol)
The impregnation mixture consisted of 95% low-molecular-weight silicone P-27 (relative molecular weight of 6400 to 6600; content of hydroxyl groups 0.7-0.8 %; kinematic viscosity at 20°C 50 - 60 cSt), with 5% content of a cross-linker agent P-27B (tetraethoxysilane). Impregnation was carried out at +20°С while lowering pressure in the vacuum chamber and maintaining a rapid boil. To prevent excess volatilization of the cross-linker (P-27B), pressure was not lowered below 30 mm Hg. Room temperature specimen curing was by spraying the surface with 30% Catalyst P-27A (dibutyltindilaurate).
The impregnation process was controlled by measuring pressure in the vacuum chamber with a pressure gauge and watching the bubbling intensity of the intermediate solvent on the silicone-mix surface. In addition, acetone vapors passing through the vacuum pump were cooled to -25°С and condensed in a cold reservoir. The quantity and density of the condensate were measured. In both experiments impregnation was judged complete after acetone collection had stopped.
Specimen shrinkage was measured by submerging the specimen cores and intestine segments in a graduated cylinder filled with the appropriate liquid (water, acetone or polymer) and recording the displaced liquid volume. The volume of the cylindrical cores and intestine segments was measured four times: after cutting, after dehydration and degreasing, after impregnation and after curing. Relative shrinkage was expressed as a percentage, and calculated according to the formula:
(Initial volume - volume after plastination stage)/initial volume x 100 = % shrinkage
The duration of impregnation and curing for experimental tissue cores from both cold and room temperature experiments are shown in Table 1.
| Cold Temperature | Room Temperature | ||
| Impregnation | 54.5 ± 6.56 | 33.7 ± 4.31 | |
| Curing including:
Draining Catalyzing Sum |
38.7 ± 4.82 8.1 ± 2.79 47.2 ± 5.24 |
8.2 ± 2.48 24.7 ± 4.34 32.4 ± 4.86 |
|
| Total hours | 101.7 ± 7.85 | 66.1 ± 6.89 | |
| Table 1. Average duration in hours of impregnation and curing stages for tissue cores ( ± SD; hours) | |||
Cold temperature impregnation time of core samples averaged 54.5 ± 6.56 hours, whereas room temperature impregnation time was 1.62 times shorter (p < 0.05). Draining excess silicone after RT impregnation took 8.2 ± 2.48 hours only, which was 4.72 times faster than for CT (p < 0.05); but catalyzing (gas hardening/curing) in the CT was dramatically decreased in time, so the overall duration of the CT curing stage was 1.46 times shorter (p < 0.05) compared to the RT curing. However, the total duration of the two cold temperature plastination stages proved to be 1.54 times longer than that of room temperature plastination (p < 0.05).
Cold temperature impregnation of large plastinates was quite variable from 10 days to six weeks, whereas room temperature impregnation time was from 5 days to 4 weeks. Impregnation was judged complete when bubbles diminished and no acetone was collected in the cold reservoir from the cooled (-25°С) pump exhaust.
The silicone impregnation-mix for the RT technique, because of its low viscosity, drains very easily from impregnated hair, fur, and feathers, which is a large time-saver when using this method on hair, fur, or feathered specimens. In contrast, the polymer-mix for the CT technique drains much more slowly after removing the specimens from the impregnation bath, and stays in hair, feathers and fur. When this silicone started to cure it was difficult to remove, so animal and bird specimens with hair, fur or feather looked less attractive after the CT process.
The average shrinkage for different tissue samples in both plastination techniques is summarized in Table 2.
| Cold temperature shrinkage % |
Room temperature shrinkage % |
||
| Liver | 22.4 ± 3.97 | 15.5 ± 3.05 | |
| Kidney | 28.3 ± 5.68 | 19.6 ± 4.11 | |
| Heart | 15.8 ± 4.52 | 12.7 ± 2.34 | |
| Brain | 46.0 ± 8.19 | 28.5 ± 5.56 | |
| Intestine | 17.1 ± 5.95 | 13.2 ± 3.27 | |
| Average | 24.3 ± 2.42 | 16.2 ± 1.49 | |
| Table 2. Volume changes of various tissue cores from fixation to after curing in cold and room temperature plastination ( ± SD; %) | |||
According to our data, at the end of curing, shrinkage was less in the experimental pieces plastinated via the room temperature protocol. The average difference in shrinkage calculated for all pieces plastinated by the RT technique (16.2 ± 1.49 %) was 1.5 times less, and statistically significantly different from that of the CT process (p < 0.05). Brain samples demonstrated maximal shrinkage (46.0 ± 8.19%) probably because they were defatted together with other tissue cores in room temperature acetone.
Sagittal head sections and non-dissected fetuses (same age and size), plastinated with both techniques, gave the opportunity to compare shrinkage visually. The cold plastinated head specimens had a larger subdural space when compared to room temperature plastinates (Fig. 1).
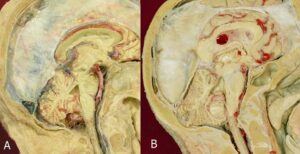
Figure 1. Median sagittal view of brain stem in human head: A - cold temperature technique; B - room temperature technique.
The cold temperature plastinated hollow organ specimens and body part specimens were more flexible and elastic after curing than those made with the room temperature technique, which produced specimens that were harder and more fragile. The most elastic and flexible specimens were made using the CT protocol (first set of experiments) which were not exposed to the cross-linking agent vapors, but it took much longer for them to cure.
The room temperature technique silicone polymerized only on the outer surface of the specimen while it remained fluid inside after initial application of catalyst. This allowed small amounts of uncured silicone to ooze from the deeper layers, especially in whole-body specimens, for several weeks after curing. For that reason, repeated catalyst application was required to complete hardening, which lengthened the hardening stage for whole body specimens. This phenomenon was not always observed with smaller specimens (body parts or organs) and was not observed at all with the cold temperature technique. When the whole plastinated organs like liver and whole brain were cut after two years of curing, fluid (non-polymerized) silicone deep inside the plastinated specimens was observed with the RT protocol. All specimens from the CT protocol had only cured polymer inside, but the depth of silicone penetration for these specimens was visibly less than for specimens produced via the RT protocol.
Room temperature-impregnated fur and feather-covered specimens yielded life-like hair and feathers with minimal specimen manicuring, while cold-impregnated fur and feather specimens required many hours of manicuring to yield near life-like/unmatted specimens. Reptile and fish RT specimens yielded more natural-appearing specimens than specimens impregnated with the cold temperature products. Fungi/mushrooms seemed to have similar results when using either technique.
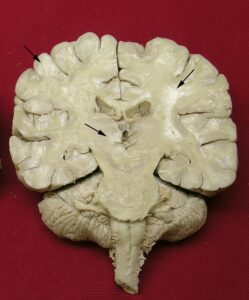
Figure 2. Frontal section of human brain. White spots are indicated by arrows - cold temperature technique.
One of the properties of the cross-linking agent tetraethoxysilane, is hydrolyzation with water. As a result, the interaction between the cross-linking agent S6 and air moisture occasionally leads to white spots/precipitate on the surface of the cold temperature technique specimens (Fig.2).
With the room temperature plastination technique, in specimens with more than 3% water content (which means inadequate dehydration), the cross-linking agent (P-27B) interacts with the water and forms small firm crystals on the specimen surface (Fig. 3).
We had to purchase an additional freezer for the vacuum chamber to impregnate specimens with the CT protocol and keep the impregnation silicone-mix in the freezer at all times even when not impregnating. We noticed that when the CT silicone-mix had been stored at -25°C for more than 6 months it became more viscous and could no longer be used. In addition to that, we had to produce a special chamber equipped with a fan,

Figure 3. Small crystals from cross-linker (P-27B) on cerebellum surface - room temperature technique.
an aquarium pump and desiccant for curing specimens with the CT protocol. There was no need for such equipment for curing according the room temperature protocol.
When silicone prices were compared in the Russian and USA markets, silicone compounds for the room temperature protocol were from 10 to 20% less than the products for cold temperature plastination.
The same silicone chemicals are combined in different sequences for cold and room temperature plastination. Yet each combination requires a different methodology for impregnation and curing, as well as handling and storage of polymer, and each yields specimens with a difference in features and quality. Many of these findings correlate with previous study results (von Hagens et al., 1987; Henry et al., 2001; Smodlaka, 2005). Traditionally, silicone polymer used for the standard cold temperature technique (S10) contains silicone with a higher molecular weight, so it is more viscous. This CT silicone mixture penetrates into tissue depths relatively slowly, which, in turn, makes the impregnation stage longer. As a result, impregnation using the cold temperature protocol is 1.62 times longer than impregnation with the less viscous room temperature products.
Compared with the CT technique, RT plastinated specimens drain 4.7 times quicker after being taken out from the impregnation-mix. This saves time and allows the application of catalyst several hours after impregnation. However, catalyzing with the RT technique takes about 3 times longer than gas hardening with the CT protocol because: 1. When the RT catalyst reacts with polymer-mix it may produce a thin cured layer outside the specimen, and 2. Sometimes it requires repeated application of the catalyst.
In spite of longer catalyzing, the overall duration of the whole curing stage (draining and catalyzing), with the room temperature technique is 1.46 times shorter than with the cold temperature protocol. Total duration of impregnation and curing stages with CT is 1.54 times longer than with RT. This correlates with other study results (Henry, 2002a) where a shorter plastination time was reported for low-molecular-weight siloxane compounds. Also, the less viscous room temperature impregnation-mix drains easily from hair/fur/feather-covered specimens, which allows them to look more natural.
Another plus for the room temperature silicone impregnation-mix is that it can sit for a long time without becoming more viscous until the catalyst is applied. Therefore, hair, fur and feathers can be allowed to drain for a long time and the polymer will not commence to cure as is the case with the cold temperature impregnation-mix. Hence, if the CT impregnation-mix is not removed within one or two weeks, it is extremely difficult to remove excess polymer from the hair which results in a matted hair/fur coat.
Specimen shrinkage with the RT protocol was 1.5 times less than with CT, which is explained by less viscosity which results in higher penetration of the room temperature plastination-mixture with low-molecular-weight silanol (Henry, 2002b). It allows more polymer to impregnate the specimen, preserving its volume and shape. Higher penetration of low-molecular-weight silicone (room temperature) can be used for impregnating dense archaeological objects and longtime formalin-preserved specimens with good results (von Hagens et al., 1987).
One of the major advantages of the cold temperature plastination products is the ability to make more flexible and elastic specimens, because the cured silicone used with this technique has higher molecular weight and higher extension coefficient than the silicone used with the room temperature technique. Another advantage of the CT technique is the possibility to vary flexibility and elasticity of plastinated specimens during the curing stage by altering exposure time to S6. Specimens without exposure to hardening vapors of S6 are the most elastic and flexible. Plastinated specimens made with the room temperature techniques have harder and firmer surfaces. In the RT process, more flexible and elastic specimens can be produced by decreasing the percentage of the cross-linking agent (P-27B) in the silicone impregnation-mix, although this increases the duration of the hardening stage and requires larger amounts of the catalyst (P-27A).
Multi-year observation of cold temperature plastinated specimens demonstrated a tendency towards surface deformation, especially in large skeletal muscle insertion sites (gluteal region, back and pectoral muscles). This phenomenon was observed mostly in gross whole-body specimens. It is likely that this happened because the cross-linking agent (S6) did not penetrate deep enough inside a large specimen and some of the silicone-catalyst mixture was left unhardened. Eventually, slow polymerization of the mixture results in stress within the tissue and slight long-term shrinkage of large specimen surfaces.
The silicone impregnation-mix for the CT protocol (first set of experiments) produces an endothermic reaction. This reaction, which lengthens polymer chains, is accelerated as temperature rises. This reaction is decelerated by lowering the temperature. Even when the CT impregnation-mix is stored in a freezer at -25⁰ С, the reaction still proceeds slowly and the mix gradually becomes more viscous. When this partly-cured mixture is used for impregnation, it cannot penetrate as well as the more fluid room temperature mix. For good penetration of such viscous polymer-mix, impregnation time must be prolonged. For this reason, monthly checks of the cold temperature impregnation-mix is recommended with timely replacement of some of the viscous silicone-mixture to ensure penetration of polymer-mix. When the polymer is too thick, it will take much time and effort to remove the polymerized silicone-mixture from the vacuum chamber. Lining the chamber with a polyethylene film will help protect the inside of the chamber and facilitate removal of viscous polymer.
Another challenge with the room temperature technique is that the cross-linker (P-27B) vaporizes from the impregnation-mixture when the pressure nears zero. It may crystallize in the vacuum pump and impair its function. Therefore, frequent changing of the pump oil is recommended.
To conduct cold temperature plastination, an extra freezer is needed to house the vacuum chamber with the silicone reaction-mixture. In addition to that, a special chamber with an air/aquarium pump is helpful for vaporizing the S6 during the hardening process. These increase laboratory set-up expenditures, as well as increasing electricity usage and cost because of the extra freezer and the longer vacuum pump operating time during the impregnation stage. The cross-linking agent S6, which is used as a hardening component with the cold technique, can cause eye conjunctiva irritation. Therefore, it is advisable to allow recently cured specimens to air out.
Catalyst P-27A, used for room temperature curing, is an organo-tin compound with low toxicity. Respirators should be used when the specimen surface is sprayed with the catalyst, and airtight bags or wrapping specimens in foil should be used while RT specimens are curing (Henry, 2007b; Raoof et al., 2007). It is also necessary for the room being used for hardening to be equipped with an effective ventilation system, to ensure a proper exchange of room air with fresh air.
In summary, experiments reported here demonstrated that specimens plastinated with the cold temperature technique are more flexible and elastic, but this process takes longer and specimens tend to shrink more. This technique is preferable for plastination of hollow organs of the gastrointestinal tract, lungs and the whole body, especially when the goal to make specimens for exhibition. Room temperature plastination is more economical to set up the laboratory, and allows production of good quality specimens in a shorter period of time. Low-molecular-weight silicone, used initially for room temperature impregnation, improves penetrability and makes it possible to reduce specimen shrinkage and surface deformation. The RT protocol can be recommended for brain (for best brain specimens cold dehydration with no degreasing is recommended), parenchymatous organs, parts of the body, fetus, fur/hair/feather-covered specimens, and reptiles & fish. This technique is also preferable for plastination of formalin-fixed specimens, as well as for archaeological and fossil objects.
Bickley НС, von Hagens G, Townsend FM. 1981: An improved method for the preservation of teaching specimens. Arch Pathol Lab Med 105:674-676.
Bickley HC, Donner RS. Walker AN. Jackson RL. 1987: Preservation of tissue by silicone rubber impregnation. J Int Soc Plastination 1(1):30-39.
https://doi.org/10.56507/XVDP9663
Chaynes P, Mingotand A. 2004: Analysis of commercial plastination agents. Surg. Radiol. Anat. 26, N3:235-238
https://doi.org/10.1007/s00276-003-0216-9
de Jong K, Henry RW. 2007: Silicone Plastination of Biological Tissue: Cold-temperature Technique Biodur S10/S15 Technique and Products. J Int Soc Plastination 22:2-14
https://doi.org/10.56507/ZLMJ7068
Glover RA, Henry RW, Wade RS. 1998: Polymer preservation technology: Poly-Cur. A next generation process for biological specimen preservation. Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J lnt Soc Plastination 13(2):39.
Glover R. 2004: Silicone plastination, room temperature methodology: Basic techniques, applications and benefits for the interested user. Abstract presented at The 12th International Conference on Plastination, Murcia, Spain July 11-16, 2004. J lnt Soc Plastination 19:7.
Henry RW. 1987: Plastination of an integral heart-lung specimen. J Int Soc Plastination l(2):20-24.
https://doi.org/10.56507/KQKI2988
Henry RW. 1995: Principles of plastination - dehydration of specimens. Abstract presented at The 7th International Conference on Plastination - Karl-Franzens-University, Graz, Austria. July 1994. J Int Soc Plastination 9(1):27.
Henry RW. 1998: Principles of plastination. Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J Int Soc Plastination 13(2):27.
Henry RW. 2007a: Silicone Plastination of Biological Tissue: Cold-temperature Technique North Carolina Technique and Products. J Int Soc Plastination 22:15-19
https://doi.org/10.56507/DGZJ6845
Henry RW. 2007b: Silicone Plastination of Biological Tissue: Room-temperature Technique North Carolina Technique and Products. J Int Soc Plastination 22:26-30
https://doi.org/10.56507/FSNZ3092
Henry RW. 2008: Room-temperature impregnation with cold temperature silicone products. Abstract presented at The 14th International Conference on Plastination -Heidelberg and Guben, Germany, July 20-26, 2008. J Int Soc Plastination 23:40
Henry RW, Reed RB, Henry CL. 2001: "Classic" silicone processed specimens vs "New formula" silicone plastinated specimens: A two year study. Abstract presented at The 10th International Conference on Plastination, Saint-Etienne, France, July 2-7, 2000. J Int Soc Plastination 16:33.
Latorre R, Vaquez JM, Gil F, Ramirez G, Lopez-Alhors O, Orenes M, Martinez-Gomariz F, Arencibia A. 2001: Teaching anatomy of the distal equine thoracic limb with plastinated slices. J Int Soc Plastination 16:23-30.
https://doi.org/10.56507/ACRF7155
Raoof А. 2001: Using a room-temperature plastination technique in assessing prenatal changes in the human spinal cord. J Int Soc Plastination 16:5-8.
https://doi.org/10.56507/UIHR4575
Raoof A., Henry RW, Reed RB. 2007: Silicone Plastination of Biological Tissue: Room-temperature Technique DowTM/ Corcoran Technique and Products. J Int Soc Plastination 22:21-25.
https://doi.org/10.56507/AWAC9285
Smodlaka H, Reed RB, Latorre R, Lopez-Albors O, Hervas JM, Cuellar R, Henry RW. 2006: Comparison of plastinated specimens prepared using six regimens. Abstract presented at The 13th International Conference on Plastination - Vienna, Austria, July 2 to 7, 2006. J Int Soc Plastination 21:22-23.
Starchik D. 2014: Pros and cons of Room Temperature plastination technique. Abstract presented at The 17th International Conference on Plastination Saint Petersburg, Russia July 14-18, 2014. J Int Soc Plastination 26(1):49.
von Hagens G. 1979a: Impregnation of soft biological specimens with thermosetting resins and elastomers. Anal Rec 194(2):247-255.
https://doi.org/10.1002/ar.1091940206
von Hagens G. 1979b: Emulsifying resins for plastination. Der Praparator 25(2):43-50.
von Hagens G. 1980: Animal and vegetal tissues permanently preserved by synthetic resin impregnation. US Pat No 4.205,059.
von Hagens G. 1981: Animal and vegetal tissues permanently preserved by synthetic resin impregnation. US Pat No 4.244.992.
von Hagens G. 1985: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomisches Institut I. Universilat, D-6900 Heidelberg, Germany.
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4):411-421.
https://doi.org/10.1007/BF00309677
Weiglein A, Henry RW. 1993: Curing (hardening, polymerization) of the polymer - Biodur S10. J Int Soc Plastination 7(l):32-35.
https://doi.org/10.56507/ABNZ7085