1Cátedra de Anatomía Comparada, Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, Bahía Blanca, Argentina
2Instituto de Ciencias Biológicas y Biomédicas del Sur (INBIOSUR), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Bahía Blanca, Argentina
3Cátedra de Fisiología Animal, Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, Bahía Blanca, Argentina
Biological collections are unique repositories of biodiversity. Ideally, institutions should have standardized protocols for preparation, storage, and conservation of materials, designed to minimize deterioration over time and to ensure that comparable results could be obtained from them. Eleven cleaning treatments, frequently used in scientific collections, were performed on Wistar rat femurs, consisting of burial (60 days), and enzymatic and chemical digestion. For the last two techniques, ten combinations of concentration of the agents (enzymes, potassium hydroxide [KOH]), temperature, and exposure time were tested. After treatment, bone integrity and percentage of surface covered by soft tissues were evaluated using images obtained by scanning electron microscopy. Good results, in terms of cleaning parameters (muscle and fat removal) were obtained with burial and with the KOH 10%/40 °C/2h and KOH 5%/40 °C/4h combinations; however, superficial desquamation, cracking, and porosity (parameters of bone surface damage) were observed in all cases. Other KOH combinations seemed to be less efficient to clean the surface, but the bones were better preserved. In enzymatic treatments, bone integrity was less affected but more residues persisted; the amount of tissue remaining appears to be related to temperature (treatments at 70 °C were more effective than at 25 °C). Damage caused by burial and KOH coincided with that observed by other authors, although enzymatic treatments left greater amounts of tissue than previously reported. The preliminary information gathered provides a starting point to implement conservative cleaning of skeletal material and will surely constitute an important advance for the establishment of protocols in biological collections.
bone cleaning techniques; bone integrity; mammalian bone preservation; skeletal material; specimen preservation
Lic. Albertina I. Popp, Departamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur, San Juan 670, B80000ICN, Bahía Blanca, Argentina. E-mail: albertina.popp@uns.edu.ar
![]()



Biological collections are unique repositories of biodiversity, and their importance has been revalued in recent years, both globally and locally (Vaught and Henderson, 2011; Kemp, 2015; Dunnum et al., 2017; Funk, 2018; Cook et al., 2020). In Argentina, these institutions house more than 60,000 mammalian specimens dating from the mid-nineteenth century. The existence of these biological materials is of enormous scientific value, since they provide useful contemporary and historical samples for different investigations (Moritz et al., 2008; Rubidge et al., 2012; Rowe et al., 2015; Di Euliis et al., 2016; Dunnum et al., 2017; Cook et al., 2020), and constitute important sources for the description of organisms, their origin, evolution and interrelationships (Suarez and Tsutsui, 2004; Wandeler et al., 2007; Schiaffini et al., 2013; Carrion-Bonilla and Cook, 2020). In addition, these repositories are of great importance in the academic field, since they constitute a regular source of consultation for teachers and students. Finally, the specimens located there constitute important biological samples for studies on the conservation of genetic diversity, since they allow the detection of possible loss in such a diversity (Smulders et al., 2003; Dures et al., 2019).
Initially, the preservation of specimens in biological collections had the purpose of exhibiting 'curiosities' and was only possible with dry inert materials (Reid, 1994). In the mid-1600´s with the use of fluid preservation, it became possible to preserve moist, soft biological material (Simmons, 2014). In recent years, with the rapid development and improvement of powerful tools that look at a microscopic or molecular scale, the requirements of modern specimens have changed. Nowadays it's imperative to find effective solutions to clean and preserve biological material for both morphological and molecular use (Brown, 1999; Wandeler et al., 2007; Miller et al., 2020). Even if researchers who habitually use materials from natural collections have started studies to assess how field collection techniques, cleaning and preservation practice affects the condition of the specimens, no effective transfer for museum workers is done (Carter, 2003; Zimkus and Ford, 2014; Nakahama 2020).
Within the wide range of existing methods for obtaining biological materials, preparation of vertebrate skeletons is the one that offers the greatest number of alternatives. However, the damage produced by conventional preparation techniques (boiling, dermestids, enzymes and hydrogen peroxide, for example) of bones could affect not only the superficial layers of these elements (appearance of cracks, peeling, holes and increased porosity) but also their histological structure, leading in the most severe cases to the deformation or even to the disintegration of the materials (Carter, 1999; Fernández-Jalvo and Marín Monfort, 2008; Hartnett et al. 2011; Leeper, 2015; Thompson, 2015; Botero-González and Agudelo, 2019). Given the current increasing value of bone material deposited in biological collections (Wandeler et al. 2007, Burrell et al. 2015, Pacheco et al. 2022) it´s now important to review the status of commonly used museum methods of specimen cleaning, in order to understand how these processes can be improved. So, the aim of this study was to perform a preliminary and qualitative evaluation of the effect of different bone preparation techniques in terms of cleaning and surface preservation.
The evaluation of the effects of different preparation techniques was carried out using femurs from 90-day-old female Wistar rats (n=11; total weight: 203.6 ± 4.6 g). Samples came from the discard of control animals (not subjected to chemical treatments or infection with pathogens), from ongoing research projects in the laboratories of the Instituto de Ciencias Biológicas y Biomédicas del Sur (INBIOSUR-CONICET), and the Departamento de Biología, Bioquímica y Farmacia (BByF, UNS), based on a protocol approved by the Comité Institucional para el Cuidado y Uso de Animales de Experimentación (CICUAE-BByF-UNS, Protocol No. 181/2021).
Animals were euthanized by CO2 inhalation and subsequently subjected to dissection to isolate the hindlimbs. All the samples were prepared by the same operator, starting with the complete removal of the skin. After recording the weight of the skinned hindlimbs (Acculab V-121; 0.01 g), the fat and muscles were carefully removed using scissors and scalpels, trying not to touch the bones to avoid their mechanical damage (Fig. 1). Special care was taken to leave a similar amount of soft tissue attached to the bones in all samples (40-42% of the initial mass), which was ensured by weighing them again.
The samples obtained were subjected to eleven treatments (Table 1). Burial was performed by placing samples within individual nylon mesh bags in loamy soil (15 cm deep) without artificial irrigation. For the digestion-based treatments, different concentrations of the agents (enzymes, EZ; potassium hydroxide, KOH), temperature, and exposure time were tested. Solutions were prepared using distilled water. Treatments at higher temperatures were conducted with a laboratory oven, using containers covered with aluminum foil to prevent evaporation. Enzymatic digestion was carried out using commercial enzyme-based laundry detergent (Skip® Bio-Enzymes Liquid Soap). The decision to use commercial detergents was based on previous studies, which reported results similar to those of traditional enzymes (papain, pepsin, pancreatin, trypsin) avoiding the high costs and the irritating odors associated with these substances (Mooney et al., 1982; Mairs et al., 2004; Austin and Fulginiti, 2008; Leeper, 2015). Taking into account the bone sizes, and according to results obtained in other mammalian species (Ossian, 1970; Mooney et al., 1982; Mairs et al., 2004; Leeper, 2015), four combinations were tested for enzymatic digestion (Table 1). For KOH treatments, and based on previous reports (Miller and Tarpley, 1996; Botero-González and Agudelo, 2019), six combinations were performed (Table 1). Upon completion of the treatments, the recovered bones were thoroughly washed with tap water, removing only the loosely soft tissue attached to the bone (tissue firmly attached to the bone was not removed) with a soft brush, and then left at room temperature until they were completely dry.
| Weight of the hindlimbs without skin
(mean ± SD; g) |
Weight of the hindlimbs after soft tissue removal
(mean ± SD; g) |
Treatment |
|
|
||
| 10.8 ± 0.5 | 4.4 ± 0.2 | Burial (60 days, at 15 cm deep in loamy soil) |
| EZ 10%/2h/70 °C | ||
| EZ 10%/70h/25 °C | ||
| EZ 15%/2h/70 °C | ||
| EZ 15%/70h/25 °C | ||
| KOH 5%/1h/25 °C | ||
| KOH 5%/2h/25 °C | ||
| KOH 5%/1h/40 °C | ||
| KOH 5% /2h/40 °C | ||
| KOH 5%/4h/40 °C | ||
| KOH 10%/2h/40 °C | ||
|
|
||
For the study, we analyzed only the proximal segment of each femur. For that purpose, a section comprising the epiphysis plus half of the diaphysis was isolated using a dental drill. The samples were processed and photographed by scanning electron microscopy (SEM LEO EVO 40 XVP-EDS OXFORD X-MAX 50). All bone segments were analyzed qualitatively in terms of
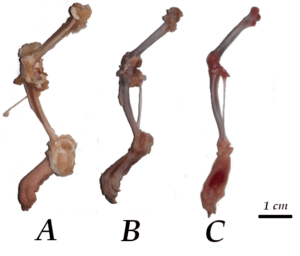
Figure 2. Examples of different levels of soft tissue removal (A: poor; B: intermediate; C: good) on the proximal section of rat femurs (for detailed explanation see text)
preservation (absence of signs of damage such as superficial desquamation, cracking, and porosity) and cleaning (amount of bone surface without soft tissue remnants) parameters. To evaluate the latter, photographs of identical magnification (25X) were selected, and a 1x1 cm grid was superimposed on each of them in order to account for the surface of bone (%) covered by
these residues. To properly compare the results, the same bone region was considered in all cases, consisting of the portion of the proximal epiphysis that included the femoral head, the neck and the entire greater trochanter. Organic remnants protruding outward from the bone surface were not considered. According to the percentage of bone surface occupied by soft tissue remnants, the results of the treatments were classified into three levels (Poor; 51-100%; Intermediate: 21-50%; Good: 0-20%; Fig. 2).
The best results in terms of soft tissue removal were obtained by burial, since the recovered bones were completely cleaned (Table 2).
| Effectiveness | Treatment | % Soft Tissue Remnants |
| Poor | EZ 10%/70h/25 °C | 70 |
| KOH 5%/1h/25 °C | 80 | |
| EZ 15%/70h/25 °C | 84 | |
|
|
||
| Intermediate | KOH 5%/ 2h/40 °C | 23 |
| EZ 15%/2h/70 °C | 31 | |
| KOH 5%/1h/40 °C | 45 | |
|
|
||
| Good | Burial | 0 |
| KOH 10%/2h/40 °C | 4 | |
| KOH 5%/4h/40 °C | 5 | |
| KOH 5%/2h/25 °C | 19 | |
| EZ 10%/2h/70 °C | 20 | |
|
|
||
However, there was a considerable deterioration of the bone surface. The entire osseous fragment analyzed presented symptoms of general weakening (Fig. 3 A), with a high degree of porosity and cracking, as well as important and macroscopic fissures at the base of the femoral head and the greater trochanter (Fig. 3B). Several grooves were also observed, especially at the base of the head (Fig. 3C).
The KOH 10%/2h/40 °C and KOH 5%/4h/40 °C treatments gave good results and were similar to burial, in terms of cleaning (Table 2 and Fig. 4), retaining only a small amount of tissue attached mostly to the greater trochanter, which is the insertion site for some of the hip rotator muscles, M. obturator externus, M. obturator internus and M. gemellus (Charles et al., 2016). However, the deleterious effect concerning the surface integrity was important, since a high degree of desquamation and porousness was observed. For the first case (KOH 10%/2h/40 °C; Fig. 4A, B, C), the highest damage was observed at the trochanteric fossa. In the KOH 5%/4h/40 °C treatment (Fig. 4D, E, F), an abnormal and generalized porosity was observed in the entire portion of the bone analyzed, as well as some areas of osseous delamination. In this case, although the temperature was the same and concentration was half of that in the other KOH treatment, the deterioration was higher, which indicated that the time of exposure could be a key factor for bone integrity when this substance is used.
With the other KOH combinations, the degree of cleaning obtained was substantially lower (Table 2), with the soft tissue remnants completely occluding the trochanteric fossa and covering the trochanters in some cases (Fig. 5). Although scattered cracks and some degree of porosity and desquamation were observed over the clean areas of the samples, the real effect of the treatments on bone integrity could not be elucidated because the higher proportion of fat and muscle prevented visualization of the entire surface.
The enzymatic treatments (Fig. 6) generally produced less damage to the bone surface than burial and exposure to KOH, considering that no cracks or peeling were observed in any of the clean bone areas, and that a conspicuous degree of porosity was only confirmed in the most aggressive combination (EZ 15%/2h/70 °C; Fig. 6D). However, the results in terms of cleaning seemed to be worse, since to achieve a removal of soft tissues greater than 50% it was necessary to subject the material to 70 °C (Table 2). In the less efficient enzymatic treatments (those conducted at 25 °C), soft tissue remnants not only occupied most of the epiphysis but also extended over the proximal portion of the diaphysis, and were firmly attached to the bone surface.
The treatments tested in this study are frequently used in scientific collections. The results obtained were mixed in terms of cleaning and conservation of the bone surface, varying in the amount of fat and muscle retained, in the degree of desquamation and porosity, and in the appearance of micro- and macroscopic cracks. Similar results were reported by Fernández-Jalvo and Marín Monfort (2008) for museum samples of both modern and fossil bones.
In terms of cleaning, burial was the treatment that removed all the soft tissue, but the degree of damage was considerable; this was especially marked at the level of the epiphysis, with severe fissures appearing at the base of the femoral head and at the greater trochanter. Given that we worked with young rats, and that the fractures were located at the place occupied by the epiphyseal plates, it is postulated that this damage could be due to chondrolysis caused by microbial attack to the growth cartilage. Necrosis and chondrolysis caused by bacteria and fungi have been reported both in vivo and in vitro, for various types of osteoarticular diseases in birds and mammals (Daniel et al., 1973, 1976; Smith et al., 1987; Wideman and Prisby, 2013; Zimmerli, 2015; Scher et al., 2016; Alder et al., 2020). There were also signs of damage in the form of grooves on the bone surface, which could be due to the action of animals that are part of the soil mesofauna, some of which are capable of producing chewing marks on the bone surface with their powerful jaws (Fernández-Jalvo and Marín Monfort, 2008).
Of the agents tested for digestion treatments, the ones that used KOH appear to be more effective in removing soft tissue, but the bone surface showed signs of deterioration (porosity, superficial desquamation, and cracking). Although in all combinations of concentration-temperature-exposure time, the bone maintained its integrity without becoming brittle, the increase in porosity could represent an augmented area where microorganisms can act, damaging the bone structure in the long-term period (Jans et al., 2004). Since the degree of deterioration is potentially related to exposure time (Steadman et al., 2006, Leeper, 2015), this factor must be strictly controlled.
Cleaning treatments with enzyme-based laundry detergent seems to produce less surface damage but leave considerable amounts of soft tissue attached to the bone. In our treatments to achieve removal levels comparable to those of KOH, it was necessary to subject the material to a considerably higher temperature (70 °C) or to an extremely long exposure time (70 hours). Both conditions can cause loss of bone microstructure, by denaturation of collagen in the first case (causing increased porosity, deformation, and alteration of bone microstructure), and by bacterial proliferation and attack in the second (Mori, 1970; Fenton et al., 2003; Fernández-Jalvo and Marín Monfort, 2008).
Our preliminary study reveals several aspects to consider in order to obtain clean bones preserving their surface from chemical, physical and/or biological deterioration. Treatment with KOH in the laboratory oven is the most practical for bone cleaning, since a large amount of skeletal material can be easily prepared with minimal effort and in a short period of time. However, under this cleaning method, deterioration of the bone surface at the macroscopic level is evident, which probably leads to the deterioration of skeletal elements in the long term.
Burial is also a processing technique that involves little work for the curator, but the degree of bone damage is very high. The less densities, smaller dimensions and also the presence of smaller crystals of hydroxyapatite in juvenile bones (compared to those of adults), constitute characteristics that make these bones more susceptible to destruction in the soil (Mays, 2021). Therefore, it could be better considered for adult specimens, or large species where other methodologies are difficult to apply (Leeper, 2015).
Our results are the first step in establishing guidelines that help in skeletal preparations, but analyses of more treatment combinations (with their replicas) that allow maximizing the cleaning of the material minimizing its damage, as well as evaluating their effect on the histological structure and on the conservation of DNA, are needed. The information obtained from the study of those characteristics will constitute a valuable tool to develop and implement conservative cleaning osseous material, and will surely constitute an important advance for the establishment of protocols in biological collections.
Acknowledgments
We are thankful to Mg. Cecilia Ayesta for processing the samples for electron microscopy. This work was funded by CONICET (PIP 11220200101668CO), ANPCyT (PICT 03298/2020) and SGCyT-UNS (PGI 24/B233). Financial support from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) through a PhD fellowship to AIP is also acknowledged.
Alder KD, Lee I, Munger AM, et al. 2020: Intracellular Staphylococcus aureus in bone and joint infections: A mechanism of disease recurrence, inflammation, and bone and cartilage destruction. Bone 141: 115568.
https://doi.org/10.1016/j.bone.2020.115568
Austin D, Fulginiti L. 2008: The forensic anthropology laboratory in a medical examiner setting. In: Warren MW, Walsh-Haney HA, Freas LE, editors. The Forensic Anthropology Laboratory. Florida, USA: CRC Press, Taylor and Francis, p 23-46.
https://doi.org/10.1201/9781420004021.ch3
Botero-González D, Agudelo M. 2019: Comparison of macerations with dermestid larvae, potassium hydroxide and sodium hypochlorite in Wistar rat crania. Anatomy 13: 149-154. https://dergipark.org.tr/tr/download/article-file/1041892.
Brown TA. 1999: Genetic material. In: Care and Conservation of Natural History Collections (Eds. D. Carter and A.K Walker), pp. 133-138. Butterworth Heinemann; Oxford.
Burrell AS, Disotell TR, Bergey CM. 2015: The use of museum specimens with high-throughput DNA sequencers. J Hum Evol 79: 35-44.
https://doi.org/10.1016/j.jhevol.2014.10.015
Carter J. 1999: Skin and bones. Biol. Curator 16: 3-8.
Carter JD. 2003: The effects of preservation and conservation treatments on the DNA of museum invertebrate fluid preserved collections. MPhil thesis.
https://doi.org/10.13140/RG.2.2.16166.93761
Carrion-Bonilla C, Cook J. 2020: A new bat species of the genus Myotis with comments on the phylogenetic placement of M. keaysi and M. pilosatibialis. Therya 11: 508-532.
https://doi.org/10.12933/therya-20-999
Charles JP, Cappellari O, Spence AJ, Hutchinson JR, Wells DJ. 2016: Musculoskeletal geometry, muscle architecture and functional specializations of the mouse hindlimb. PLoS ONE 11: e0147669.
https://doi.org/10.1371/journal.pone.0147669
Cook JA, Arai S, Armién, et al. 2020: Integrating biodiversity infrastructure into pathogen discovery and mitigation of emerging infectious diseases. BioScience 70: 531-534.
https://doi.org/10.1093/biosci/biaa064
Daniel D, Akeson W, Amiel D, Ryder M, Boyer J. 1976: Lavage of septic joints in rabbits: Effects of chondrolysis. J Bone Joint Surg 58: 393-395.
https://doi.org/10.2106/00004623-197658030-00018
Daniel D, Boyer J, Green S, Amiel D, Akeson W. 1973: Cartilage destruction in experimentally produced Staphylococcus aureus joint infections: In vivo study. Surg Forum 24: 479-481.
Di Euliis D, Johnson K, Morse S, Schindel D. 2016: Opinion: Specimen collections should have a much bigger role in infectious disease research and response. Proc Nat Acad Sci 113: 4-7.
https://doi.org/10.1073/pnas.1522680112
Dunnum J, Yanagihara R, Johnson K, et al. 2017: Biospecimen repositories and integrated databases as critical infrastructure for pathogen discovery and pathobiology research. PLoS Negl Trop Dis 11: e0005133.
https://doi.org/10.1371/journal.pntd.0005133
Dures S, Carbone C, Loveridge A, et al. 2019: A century of decline: Loss of genetic diversity in a southern African lion‐conservation stronghold. Divers Distrib 25: 870-879.
https://doi.org/10.1111/ddi.12905
Fenton TW, Birkby WH, Cornelison J. 2003: A fast and safe non-bleaching method for forensic skeletal preparation. J Forensic Sci 48: 274-276.
https://doi.org/10.1520/JFS2002034
Fernández-Jalvo Y, Marín-Monfort MD. 2008: Experimental taphonomy in museums: Preparation protocols for skeletons and fossil vertebrates under the scanning electron microscopy. Geobios 41: 157-181.
https://doi.org/10.1016/j.geobios.2006.06.006
Funk V. 2018: Collections-based science in the 21st century. J Syst Evol 56: 175-193.
https://doi.org/10.1111/jse.12315
Hartnett KM, Fulginiti LC, Di Modica F. 2011: The effects of corrosive substances on human bone, teeth, hair, nails, and soft tissue. J. Forensic Sci. 56: 954-959.
https://doi.org/10.1111/j.1556-4029.2011.01752.x
Jans MME, Nielsen-Marsh CM, Smith CI, Collins MJ, Kars H. 2004: Characterisation of microbial attack on archaeological bone. J Archaeol Sci 31: 87-95.
https://doi.org/10.1016/j.jas.2003.07.007
Kemp C. 2015: The endangered dead. Nature 518: 292-294.
https://doi.org/10.1038/518292a
Leeper BJ. 2015: Evaluation of current methods of soft tissue removal from bone. Ph.D. thesis. University of Pittsburgh, USA, 133 pp.
Mairs S, Swift B, Rutty GN. 2004: Detergent: An alternative approach to traditional bone cleaning methods for forensic practice. Am J Forensic Med Pathol 25: 276-284.
https://doi.org/10.1097/01.paf.0000147320.70639.41
Mays S. 2021: The archaeology of human bones. 3rd Ed, New York, Oxford: Routledge.
https://doi.org/10.4324/9781315171821
Miller DM, Tarpley J. 1996: An automated double staining procedure for bone and cartilage. Biotech Histochem 71: 79-83.
https://doi.org/10.3109/10520299609117138
Miller SE, Barrow LN, Ehlman SM, Goodheart JA, et al. 2020: Building natural history collections for the twenty-first century and beyond. BioScience 70: 674-687.
https://doi.org/10.1093/biosci/biaa069
Mooney MP, Kraus EM, Bardach J. 1982: Skull preparation using the enzyme-active detergent technique. Anat Rec 202: 125-129.
https://doi.org/10.1002/ar.1092020115
Mori JL. 1970: Procedures for establishing a faunal collection to aid in archaeological analysis. Am Antiq 35: 387-389.
https://doi.org/10.2307/278351
Moritz C, Patton J, Conro C, Parra J, White G, Beissinger S. 2008: Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322: 261-264.
https://doi.org/10.1126/science.1163428
Nakahama N. 2020: Museum specimens: An overlooked and valuable material for conservation genetics. Ecol Res 36: 13-23.
https://doi.org/10.1111/1440-1703.12181
Ossian CR. 1970: Preparations of disarticulated skeletons using enzyme-based laundry "pre-soakers". Copeia 1: 199-200.
https://doi.org/10.2307/1441999
Pacheco C, Lobo D, Silva P, et al. 2022: Assessing the performance of historical skins and bones for museomics using wolf specimens as a case study. Front Ecol Evol, 10.
https://doi.org/10.3389/fevo.2022.970249
Reid G. 1994: The preparation and preservation of collections. In: Manual of Natural History Curatorship, (Eds. G.Stanfield, J.Mathias, and G.Reid), pp. 28-69. HMSO.
Rowe K, Tingley M, Koo M, et al. 2015: Spatially heterogeneous impact of climate change on small mammals of montane California. Proc Royal Soc B 282: 20141857. https://royalsocietypublishing.org/doi/epdf/10.1098/rspb.2014.1857.
https://doi.org/10.1098/rspb.2014.1857
Rubidge E, Patton J, Lim M, Burton A, Brashares J, Moritz C. 2012: Climate-induced range contraction drives genetic erosion in an alpine mammal. Nat Clim Change 2: 285-288.
https://doi.org/10.1038/nclimate1415
Scher JU, Littman DR, Abramson SB. 2016: Microbiome in inflammatory arthritis and human rheumatic diseases. Arthritis Rheumatol 68: 35-45.
https://doi.org/10.1002/art.39259
Schiaffini M, Gabriell M, Prevosti F, et al. 2013: Taxonomic status of southern South American Conepatus (Carnivora: Mephitidae). Zool J Linn Soc 167: 327-344.
https://doi.org/10.1111/zoj.12006
Simmons JE. 2014: Fluid preservation: a comprehensive reference. Rowman & Littlefield.
Smith RL, Schurman DJ, Kajiyama G, Mell M, Gilkerson E. 1987: The effect of antibiotics on the destruction of cartilage in experimental infectious arthritis. J Bone Joint Surg 69: 1063-1068.
https://doi.org/10.2106/00004623-198769070-00015
Smulders M, Snoek L, Booy G, Vosman B. 2003: Complete loss of MHC genetic diversity in the common hamster (Cricetus cricetus) population in The Netherlands. Consequences for conservation strategies. Cons Genet 4: 441-451.
https://doi.org/10.1023/A:1024767114707
Steadman DW, DiAntonio LL, Wilson JJ, Sheridan KE, Tammariello SP. 2006: The effects of chemical and heat maceration techniques on the recovery of nuclear and mitochondrial DNA from bone. J Forensic Sci 51:11-7.
https://doi.org/10.1111/j.1556-4029.2005.00001.x
Suarez AV, Tsutsui ND. 2004: The value of museum collections for research and society. BioScience 54: 66-74.
https://doi.org/10.1641/0006-3568(2004)054[0066:TVOMCF]2.0.CO;2
Thompson MC. 2015: Preparing skeletons for research and teaching from preserved human specimens. MSc thesis. California State University, East Bay, 162 pp.
Vaught JB, Henderson MK. 2011: Biological sample collection, processing, storage and information management. IARC Sci Publ 163: 23-42.
Wandeler P, Hoeck P, Keller L. 2007: Back to the future: Museum specimens in population genetics. Trends Ecol Evol 22: 634-642.
https://doi.org/10.1016/j.tree.2007.08.017
Wideman RF, Prisby RD. 2013: Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: A translational model for the pathogenesis of femoral head necrosis. Front Endocrinol 3: 183.
https://doi.org/10.3389/fendo.2012.00183
Zimkus BM, Ford LS. 2014: Best practices for genetic resources associated with natural history collections: Recommendations for practical implementation. Collection Forum 28: 77-112.
https://doi.org/10.14351/0831-4985-28.1.77
Zimmerli W, editor. 2015. Bone and Joint Infections. From Microbiology to Diagnostics and Treatment. Oxford, UK: Wiley, 432 pp.
https://doi.org/10.1002/9781118581742