Department of Comparative Medicine, College of Veterinary Medicine, University of Tennessee, 2407 River Drive, Knoxville, 77V, 37996, USA.
To determine the effects of reduced pressure on the reaction mixture used in epoxy plastination, the components of this process, both individually and in combination, were exposed to a reduced pressure within a vacuum chamber. Production of bubbles within the reaction mixture components occurred at three different points during the experiment. The first generation of bubbles appeared with a slight decrease in pressure and most likely came from air trapped in the mixtures. The second generation of bubbles appeared when the manometer reached 10.3cm of Hg. These bubbles were released from the epoxy polymer (E12). The third generation of bubbles appeared when the manometer reached 1.0 cm of Hg. These bubbles were released from the epoxy hardener (E1). Release of these components of the epoxy reaction mixture does not affect their ability to cure.
epoxy; plastination; vacuum
R.B. REED: Telephone: 865 - 974 - 5823; Fax: 865 - 974 - 5640; E-mail: rbreed@utk.edu
![]()



Plastination of biological tissue with epoxy polymer is a unique method by which to preserve thin slices of tissue. These tissue slices may be used for teaching or research purposes (Guhr et al., 1987; Cook, 1997a; Cook 1997b; Phillips et al., 2002). The epoxy in which the specimens are preserved should be as free of artifacts as possible to enhance their usefulness to education or medical investigations. Some of the difficulties plastinators encounter when using epoxy polymer have been reported (Mathura, 1996; Weber and Henry, 1993). One artifact which commonly appears in epoxy plastinated sheets of tissue is the presence of bubbles. Air is introduced into the epoxy reaction mixture during mixing or during filling of casting chambers. Air bubbles may be removed from reaction filled casting chambers by increasing external pressure on the sides of the casting chamber (Guhr et al., 1987), teasing them out with a probe (von Hagens, 1985; Weber and Henry, 1993; Latorre et al., 2002) or drawing them out under vacuum (Weber and Henry, 1993; Skalkos et al., 1999; Latorre et al., 2002). When using the vacuum method, the reduction in pressure often appears to increase the number of bubbles in the reaction mixture. Initially, gas bubbles are removed from the casting chamber when pressure is decreased. This is followed by the manifestation of many pinpoint size bubbles which often increase in number to the point of producing a foam on the surface of the reaction mixture (Latorre et al., 2002). It is possible that decreased pressure does not just pull air from the epoxy but is also capable of removing some component of the polymer, in a gaseous form, from the reaction mixture. This study evaluated the components of epoxy plastination individually and in combination under vacuum in an attempt to establish the source of the additional bubbles.
The components of epoxy (El2) plastination were mixed at 95 parts of polymer to 26 parts of hardener to 5 parts of glass separator by weight (von Hagens, 1989). The experimental reaction mixtures were as follows: mixture 1 - E1 alone, mixture 2 - E12 alone, mixture 3 - AE30 alone, mixture 4 - E1 and E12, mixture 5 - E1 and AE30, mixture 6 - E12 and AE30, mixture 7 - E1, E12 and AE30. Each test combination was poured into 50ml glass beakers and stirred gently to avoid the introduction of excess air. Each mixture combination was produced in triplicate. The beakers and contents were placed into a vacuum chamber and the pressure was gradually decreased. Pressure was initially measured with a pressure gauge and subsequently measured with a manometer as the vacuum increased (Henry and Thompson, 1992). The experiment was conducted at room temperature (22- 23°C). The contents of the beakers were monitored for production of bubbles throughout the experiment. All beakers were left in the vacuum chamber until the point that bubble production ceased in all beakers. Mixtures were evaluated 2, 30 and 180 days following completion of the experiment to assess curing and appearance of the final products.
The beaker containing glass separator never showed bubble formation throughout the duration of the experiment. The level of glass separator in the beaker decreased gradually over time and had entirely evaporated within 12 hours.
All other combinations of reaction mixture components showed generation of small bubbles with an approximate diameter of 0.5mm or less when gauge pressure measured between 12.5 and 15.0cm Hg (Table 1). The beakers containing polymer alone and polymer with glass separator both produced bubbles of 0.5mm or less in diameter
| El | 14.2 cm Hg |
| E12 | 12.5 cm Hg |
| AE30 | - |
| E1 + E12 | 15.0 cm Hg |
| El + AE30 | - |
| E12+AE30 | 12.5 cm Hg |
| E1+E12+AE30 | - |
at 12.5cm Hg on the gauge. The beaker containing only hardener showed a generation of these same sized bubbles at 14.2cm Hg on the gauge. The beaker containing hardener with polymer showed generation of these bubbles at 15.0cm Hg on the gauge. The beaker containing hardener with separator as well as the beaker containing all three components showed bubble production so slight that it could almost be considered negligible. These small diameter bubbles persisted in the beakers, clustered around the surface of the liquids, without much change until larger bubbles were later generated which obliterated them. These initial bubbles were not captured photographically due to their extremely small size combined with the difficulties involved in photographing the beakers through the thick glass cover of the vacuum chamber.
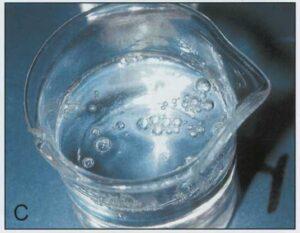
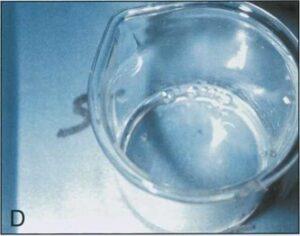
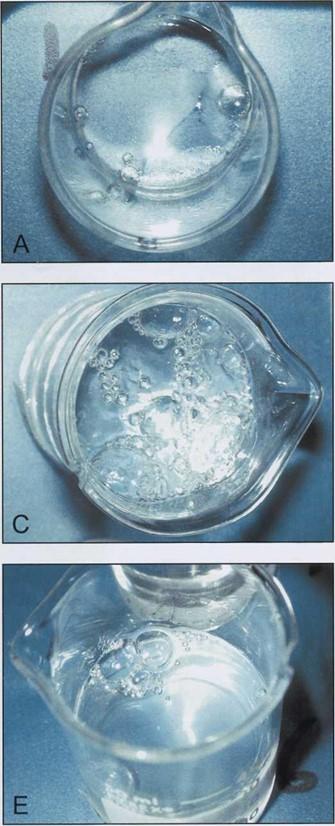
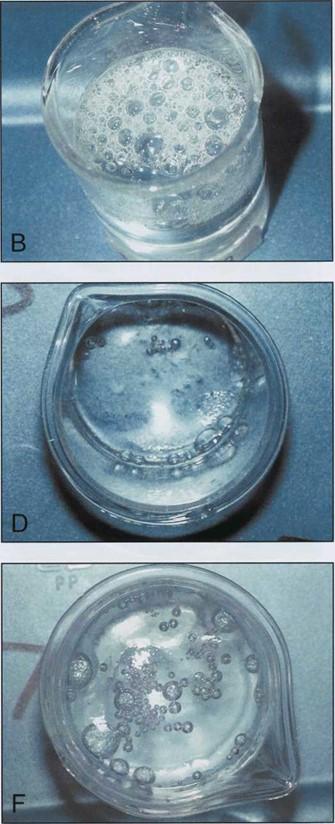
When the manometer reached 10.3cm Hg, all four beakers containing epoxy polymer began to produce bubbles measuring from 3.0 to 12.0mm in diameter (Table 2) (Figs. 1B, 1C, 1E, 1F). The two beakers in which the polymer and hardener were combined produced bubbles vigorously at 10.3cm Hg (Figs. 1C, 1F). A few bubbles were generated in the beaker containing E1 combined with AE30, however these bubbles appeared only occasionally and remained present at the surface as they were not disrupted (Fig. 1D). No bubble production occurred in the beaker containing E1 alone (Fig. 1A).
| E1 | 1.0 cmHg |
| E12 | 10.3 cm Hg |
| AE30 | - |
| E1+E12 | 10.3 cm Hg |
| El + AE30 | l.0cmHg |
| E12+AE30 | 10.3 cm Hg |
| E1+E12+AE30 | 10.3 cm Hg |
At 1.0cm Hg, the beakers containing only hardener and hardener with glass separator began to produce bubbles measuring from 2.0 - 3.0mm in diameter (Table 2) (Figs. 2A, 2D). The four beakers containing polymer continued to produce bubbles at 1.0cm Hg (Figs. 2B, 2C, 2E, 2F). The beakers containing polymer alone and polymer with hardener exhibited a marked production of gas bubbles at 1.0cm Hg (Figs. 2B, 2C).
Bubble production in the six beakers continued to the point of greatest vacuum which was 0.7cm Hg. Bubble production continued in the contents of the beakers until they gradually began to taper off between 18 and 24 hours after the experiment began. Twenty-four hours after the start of the experiment, all bubble production had ceased. Beakers were then kept at room temperature (22-23°C) on a table where they were exposed to both natural and fluorescent light.
Forty-eight hours after the experiment began, the reaction mixtures containing the polymer + hardener and the polymer + hardener + glass separator had cured and were solid. The mixture with no separator cured crystal clear while that with the separator appeared turbid. The beakers with reaction mixtures containing the polymer + hardener and the polymer + hardener + glass separator which had cured contained air bubbles on the bottom of the beakers. Forty-eight hours after the experiment began, the beakers containing hardener alone and hardener + glass separator had sticky, semi- solids present in them. Forty-eight hours after the experiment began, the contents of the beakers containing polymer alone and polymer + glass separator remained in liquid form.
 |
 |
 |
 |
 |
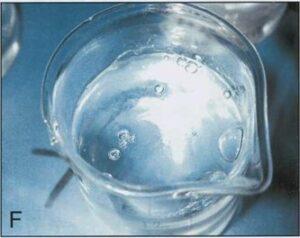 |
| Figure 1. Components of epoxy reaction mixture at 10.3 cm of Hg. El hardener (A); E12 polymer (B); El and E12 (C); El and AE30 glass separator (D); E12 an AE30 (E); E1, E12 and AE30 (F). | |
One month following completion of bubble production in the beakers, the contents did not differ from the way they appeared 48 hours post- experimentation with the exception of the beakers containing hardener and hardener plus separator showing a roughened surface to the semi-solid, sticky substance.
 |
 |
| Figure 2. Components of epoxy reaction mixture at 1.0 cm of Hg. El hardener (A); El2 polymer (B); El and El 2 (C); El and AE30 glass separator (D); E12 an AE30 (E): El. E12 and AE30 (F). | |
One hundred eighty days after the vacuum test, the contents of beakers #2 (E12) and #6 (E12, AE30) were still clear liquid. The contents of beakers # 1 (E1) and #5 (E1, AE30) were an opaque liquid with a thick film formed over the surface. The liquid in beaker #1 was almost gelatinous in consistency. The contents of beakers #4 (El, E12) and #7 (E1, E12, AE30) were hardened and clear. The cured epoxy in beaker #4 was crystal clear while that in beaker #7 exhibited a slight opaqueness.
Bubble production in the test beakers began at three different points during the experiment. The first bubbles generated in the beakers were most likely comprised of air which was dissolved in the reaction mixtures prior to their introduction into the vacuum chamber. These bubbles were not visible in the beginning of the experiment but were produced by a moderate decrease in pressure (12.5 to 15.0cm Hg gauge pressure). This initial bubble production occurred randomly across the samples with some of the beakers not showing any bubble production. This demonstrates that even moderately reducing pressure can produce more bubbles than were present before the vacuum was initiated and is apparently not linked to any one component of the reaction mixture. The fact that the beaker containing hardener with separator as well as the beaker containing all 3 components showed only very slight air bubble production when compared to the others might simply be due to a lower concentration of air in the mixtures when compared to the others prior to their placement into the vacuum chamber.
Subjecting epoxy reaction mixture to extreme reductions in pressure releases components of the mixture in a gaseous form into the casting chamber. A second wave of bubble production occurs when vacuum chamber pressure of less than 10.0cm Hg causes the release of a component of the El2 polymer in a gaseous form. A vacuum chamber pressure of less than 1.0cm Hg causes release of a gaseous component of the El hardener in a third wave of bubble production. One can be fairly certain that these gas bubbles are being released from the chemicals present in the reaction components as they were specific to the contents of the beakers as well as to two different pressure measurements. All beakers containing El2 began to show bubble production once the manometer reached 10.0cm Hg. The beakers containing El, which were not previously bubbling due to any El2 present in them as well, began to bubble at 1.0cm Hg. This shows a clear correlation between pressure and bubble production by the two components of the reaction mixture. Additionally, it is doubtful the bubbles produced after the pressure dropped to 10.3cm and lower were due to air because of the vast amount of bubbles generated.
Bubbles were not produced in the beaker containing the glass separator. Because this beaker was empty at the end of the experiment, it is obvious that the compound simply vaporized from the surface of the liquid without bubble production. The few bubbles produced at 10.3cm of Hg in the beaker containing the hardener plus glass separator were most likely glass separator bubbling up through the hardener. This can be assumed because the beaker containing only hardener produced no bubbles at the same reduction in pressure.
Release of these gas bubbles from El and E12 apparently does not affect their ability to fully cure when both are present in the same mixture. This might suggest that it is not just a portion of these chemicals which is being released but the actual vaporization of the entire compound. However, when bubble production ceased, the beakers containing El alone and El2 alone still contained liquids. Thus, vaporization of the entire product can be ruled out as the source of the second and third appearances of bubbles.
The release of these components of El and El2 produces an increase in the amount of bubbles present in the reaction mixture and decreases the aesthetics of the finished product. This is counterproductive to the employment of a vacuum chamber to remove air bubbles from the casting chambers during epoxy plastination of tissues. Thus, placing epoxy reaction mixture under vacuum to aid in air bubble removal when plastinating at room temperature should be done at less than 12.5cm Hg gauge pressure so as not to produce more bubbles than were initially present. Filling casting chambers carefully to avoid introduction of air bubbles and teasing out the bubbles which inevitably become trapped is most likely the safest approach in reducing air bubble artifacts in epoxy slices.
Adding glass separator to the reaction mixture may be responsible for the turbid streaking seen in the clear areas of some epoxy plastination slices. Further studies must be conducted to determine this definitively. The roughened surface observed on the contents of the beakers containing hardener and hardener plus the glass separator 48 hours after experiment completion was most likely due to desiccation of the semi-solid contents.
Cook P. 1997a: Sheet plastination as a clinically based teaching aid at the University of Aukland. Acta Anat 158(l):33-36.
https://doi.org/10.1159/000147907
Cook P. 1997b: Submacroscopic interpretation of human sectional anatomy using plastinated El2 sections. J Int Soc Plastination 12(2): 17-27.
https://doi.org/10.56507/XICY2283
Guhr A, Mueller A, Anton H, von Hagens G. 1987: Complete examination of mastectomy specimens using sheet plastination with epoxy resin. J Int Soc Plastination l(l):23-29.
https://doi.org/10.56507/NXYR1705
Henry R.W., Thompson J.R.: Vacuum, vacuum gauges and monometers. Presented at The 6th International Conference on Plastination -Kingston, Ontario, Canada - July 1992 J Int Soc Plastination 6(1): 10. 1992
Latorre RM, Reed RB, Gil F, Lopez-Albors O, Ayala MD, Martinez-Gomariz F, Henry RW. 2002: Epoxy impregnation without hardener: to decrease yellowing, to delay casting and to aid bubble removal. J Int Soc Plastination 17:17-22.
https://doi.org/10.56507/LZKY8224
Mathura G. 1996: Problem encountered in E12 sheet plastination technique. Presented at The 8th International Conference on Plastination - University of Queensland, Brisbane, Australia. July 1996. J Int Soc Plastination 11:13.
Phillips MN, Nash LG, Barnett R, Nicholson H, Zhang 2002: The use of confocal microscopy for the examination of E12 sheet plastinated human tissue. J Int Soc Plastination 17:12-16.
https://doi.org/10.56507/LPFJ4438
Skalkos E, Williams G, Baptista CAC. 1999: The E12 technique as an accessory tool for the study of myocardial fiber structure analysis in MRI. J Int Soc Plastination 14(1): 18-21.
https://doi.org/10.56507/ZLPM3596
von Hagens G. 1985: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. D- 6900 Heidelberg, Germany: Anatomisches Institut I, Universitat Heidelberg.
von Hagens G. 1989: Biodur™ Products: Polymers, auxiliaries and equipment for plastination. A catalog and price list. Rathausstrasse 18, Heidelberg, Germany: Biodur, p. 34-35.
Weber W, Henry RW. 1993: Sheet plastination of body slices - El2 technique, filling method. J Int Soc Plastination 7:16-22.
https://doi.org/10.56507/EZGX2343