1 Department of Anatomy, College of Veterinary Medicine, King Faisal University, Al-Ahsa 31982, Kingdom of Saudi Arabia1.
2Department of Anatomy, Faculty of Veterinary Medicine, University of Khartoum. PO Box: 32, Postal code: 13314 Shambat, Khartoum North, Sudan.
Plastination is method for long-term preservation of biological tissue, to produce dry, durable, convenient and natural looking specimens that are useful as a unique teaching aid for anatomy, pathology, radiology and surgery. The present study describes, for the first time, the plastination laboratory in the Faculty of Veterinary Medicine, University of Khartoum, Sudan. The standard Biodur ®S10 plastination technique was carried out in formalin-fixed specimens of goat and donkey. They were first dehydrated in acetone. Forced impregnation was then carried out using a vacuum chamber, and lastly, the specimens were hardened in a gas curing chamber. Plastinated specimens were long-lasting, and can be an important adjunct to traditional methods of teaching; they are also excellent museum specimens.
acetone; formaldehyde; laboratory; museum; plastination; S10; Sudan
Dr. Mohamed, A.M.A.Department of Anatomy, College of Veterinary Medicine, King Faisal University, Al-Ahsa 31982, Kingdom of Saudi Arabia. Mobile: +966530588021, email: abduseory@hotmail.com
![]()



Plastination is the method of long-term preservation of biological tissues with excellent surface details and high durability. It was developed by Dr. Gunther von Hagens in 1978 at the Heidelberg University in Germany (von Hagens, 1979). Although it is difficult to prepare a well-plastinated specimen, it is the most promising method to preserve specimens as an alternative to formalin preservation (Dawson, 1990).
In recent years, plastination has revolutionized the way in which gross anatomy can be presented to students (Latorre et al., 2007). Therefore, many Departments of Anatomy in medical colleges throughout the world started to establish plastination techniques in their own laboratories (Briggs et al., 1997; Asadi, 1998; Reina-de la Torre et al., 2004; Ali and Al-Thnaian, 2007; Suganthy et al., 2012 and Sawad and Al-Asadi, 2014)
The aim of this study was to initiate and establish S10 plastination, in the plastination laboratory in the Department of Anatomy, Faculty of Veterinary Medicine, University of Khartoum, Sudan.
The standard Biodur® silicone S10 technique for preservation of specimens in the laboratory of plastination at the Department of Anatomy, Faculty of Veterinary Medicine, University of Khartoum, Sudan, was established in 2017. The laboratory was designed according to the Plastination Technical Leaflets of Heidelberg (Von Hagens, 1986). Financial resources were obtained from the University of Khartoum, to promote new technologies in teaching anatomy in faculties of Medicine and Veterinary Medicine in Sudan.
After the plastination lab had been set up, all specimens for plastination were obtained from the Department of Anatomy as follows: kidneys, hearts, spleen and liver, from goats that had been infused with 10% formalin; and heart and whole stomach from old donkey specimens that had been fixed with 10% formalin for over a year. For preparing specimens for plastination, the standard silicone (S 10) method (von Hagens, 1979; 1986; von Hagens et al., 1987) was used. The basic steps for plastination technique are: specimen preparation, dehydration & degreasing, impregnation, and curing.
Specimens were prepared, fixed with 10% formalin at room temperature for 2 days, and then refrigerated at 4 °C for 24 hours. They were then dehydrated in cold acetone (–25 °C) with three weekly changes to minimize tissue shrinkage. After cold dehydration, the specimens and acetone bath were brought to room temperature for two days, for lipid removal. Forced impregnation was then carried out, by placing the specimen into the silicone polymer/catalyst S10/S3 mixture (100:1) (Biodur®, Germany) in the vacuum chamber at -20 °C, and gradually reducing the pressure. The final vacuum ranged between 2 and 15 mmHg, and impregnation time was four weeks.
Specimens were removed from the silicone bath, and kept on a strainer at room temperature for 24 hours. Specimens were then positioned in a gas curing chamber containing S6 (Biodur®, Germany) in a small glass container, at room temperature, for three weeks. After the gas curing step, the specimens were ready to use.
The laboratory in the Department of the Anatomy had been organized in order to host the plastination production process. The total area of the laboratory is 52 m2 (3 rooms of different sizes), with large windows, and extraction fans for providing adequate ventilation. The freezers for dehydration are located in a separate room (Fig. 1[a]) with a Bennert manometer (Fig. 1[b]) and separator for oil and solvents (Fig. 1[c]); the freezer’s compressors are located in another, adjacent, room of the laboratory. The gas curing unit (Fig. 2[a]) is located in the third room, with other necessary materials of the plastination process: stainless steel drums (Fig. 2[b]), conveyor pump (Fig.2[c]); and wire baskets, and impregnation containers (Fig. 3).
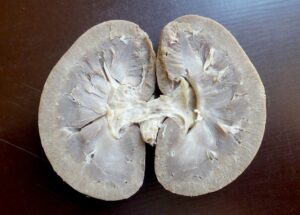
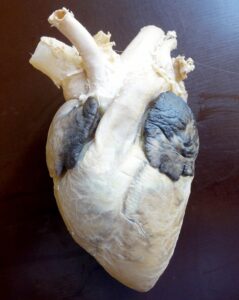
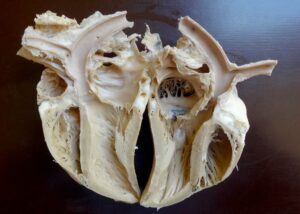
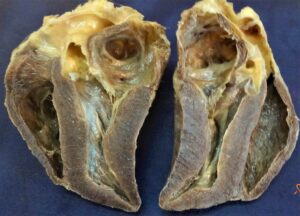
Plastinated specimens of the goat: longitudinal section of kidney (Fig. 4), liver (Fig. 5), cranio-lateral view for heart (Fig. 6), longitudinal section for heart (Fig. 7), and spleen (Fig. 8) were found to be quite similar to their natural appearance, and kept their previous morphological features, with minimal shrinkage. They were odorless, durable, non-hazardous, easy-to-handle, formalin-free, and life-like, and could be handled without a need for personal protective equipment. In contrast, the plastinated specimens of the donkey, longitudinal section of the heart (Fig. 9) and whole stomach (Fig. 10), were dark brown in color.
 |
 |
 |
 |
 |
 |
The Faculty of Veterinary Medicine at the University of Khartoum represents the first research laboratory in the Sudan where plastination has begun to improve the teaching in practical anatomy, and to reduce the exposure to toxic fumes for teachers, technical staff and students.
The preservation of anatomical specimens has been a long-standing goal of anatomists, pathologists and other medical educators (Baptista et al., 1989). In recent decades, plastinated specimens are near ideal, and are excellent for teaching gross anatomy and neuroanatomy (where routine specimens are delicate and scarce). Their anatomical structure is well preserved, and appears like a fresh specimen (Henry, 2004).
The plastination laboratory at the Faculty of Veterinary Medicine, University of Khartoum, has produced good quality plastinated specimens for the first time in Sudan. There were a limited number of unsuccessful plastinates, where the specimens changed color to dark brown, which was likely due to the old formalin-fixed specimens, as reported by Miklošová and Mikloš (2004).
The good quality plastinated specimens are used as anatomical specimens for education and for study, as predicted by Dibal et al, (2018). It is also intended to use the laboratory to train technicians in the techniques of plastination, and to encourage higher degree students in the area. Financial support for this laboratory was obtained from the University of Khartoum, Sudan, to improve the capabilities of the laboratories, and to aid new technologies in teaching anatomy. The general belief that production of plastinates is expensive needs revision. Thus, future research should target the development of fast and cost-effective techniques of plastination.
Ali AM, Al-Thnaian TA. 2007: Preservation of ruminant and equine anatomical specimens by silicone plastination. Sci J King Faisal University (Basic and Applied Sciences) 8:111- 119.
Asadi MH. 1998: Plastination of sturgeons with the S10 technique in Iran: the first trials. J Int Soc Plastination 13:15-16.
https://doi.org/10.56507/XSTD4829
Baptista CAC, Skie M, Yeasting RA, Ebraheim N, Jackson WT. 1989: Plastination of wrist: potential uses in education and clinical medicine. J Int Soc Plastination 3:18-21.
https://doi.org/10.56507/XENF9035
Briggs CA, Robbins SG, Kaegi WH. 1997: Development of an anatomical technologies laboratory. J Int Soc Plastination 12:8-11.
https://doi.org/10.56507/YRLU4811
Dibal NI, Garba SH, Jacks TW. 2018: Plastinates: possible tool for medical education in the near future: mini review. Res Dev Med Educ 7:3-7.
https://doi.org/10.15171/rdme.2018.002
Dawson TP, James RS, Williams GT. 1990: How do we teach pathology? Silicone plastinated pathology specimens and their teaching potential. J Path 162:265-272.
https://doi.org/10.1002/path.1711620314
Henry RW. 2004: Polyester plastination techniques, specific troubles and problems. Murcia, Spain, 12th International Conference on Plastination.
Latorre RM, García-Sanz MP, Moreno M, Hernández F, Gil F, López O, Ayala MD, Ramírez G, Vázquez JM, Arencibia A, Henry RW. 2007: How useful is plastination in learning anatomy? J Vet Med Educ 34:172-176.
https://doi.org/10.3138/jvme.34.2.172
Miklošová M, Mikloš V. 2004: Plastination with silicone method S 10 - Monitoring and analysis cause of failure. Biomed Papers 148: 237-238.
https://doi.org/10.5507/bp.2004.048
Reina-de la Torre F, Rodríguez-Baeza A, Doménech-Mateu JM. 2004: Setting up a plastination laboratory at the Faculty of Medicine of the Autonomous University of Barcelona. Eur J Anat 8: 1-6.
Sawad AA, Al-Asadi FS. 2014: Establishing a plastination laboratory at the College of Veterinary Medicine, University of Basra, Iraq. J Plast 26:30- 33.
https://doi.org/10.56507/YMVO2353
Suganthy J, Deepak Vinod Francis. 2012: Plastination using standard S10 technique - our experience in Christian Medical College, Vellore. J Anat Soc India 61: 44-47.
https://doi.org/10.1016/S0003-2778(12)80012-8
von Hagens G. 1979: Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 194: 247- 255.
https://doi.org/10.1002/ar.1091940206
von Hagens G. 1986: Heidelberg plastination folder. Collection of all technical leaflets for plastination. 2nd Edn. Heidelberg, Anatomische Institut, Universität Heidelberg.
von Hagens G. Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175:411-421.
https://doi.org/10.1007/BF00309677