1- Department of Anatomy, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
2- Department of Micology, Fundagdo Oswaldo Cruz, Rio de Janeiro, RJ, Brazil
Fungal contamination of plastinated specimens has not been previously reported in literature. However, during a tropical rainstorm period, last summer, we have been surprised by a massive fungal infestation of our plastinated specimens collection. The intent of this report is to discuss the probable causes, consequences and prevention of this misfortune, as well as to present an efficient, harmless and low-cost fungicide method that can be used in plastinated specimens.
Fungi, Fungicide, Fungal Contamination
Rafael Augusto Dantas Prinz, Rua Gurupi 105, Apartamento 301, Grajaii Rio de Janeiro - RJ, Brazil, CEP: 20561-100. Tel: 55 21 290 0587 / Fax: 55 21 562 6394. Email: prinz@netrio.com.br
![]()



Last summer, after a tropical rainstorm period, there was an overflow in our Plastinated Specimens Facility. After that misfortune, progressive, fast growing white, green and black spots could be seen over almost all the plastinated specimens (figure 1) as well as over the wooden shelves (figure 2). Before the overflow, the specimens were stored in a low humidity environment (Correia et al., 1998) and all have been plastinated according to the standard S10 technique (von Hagens, 1985).
Mycological analysis revealed fungal contamination by several different species. Therefore, we tried to develop a fungicide process that would be efficient, inexpensive and harmless for the plastinated specimens.
Mycological analysis of plastinated specimens
Eight specimens that had the largest surface infestation (1 heart, 1 specimen with the muscles of rotator cuff, 1 kidney, 1 abdominal sagital section, 1 abdominal transverse section, 1 cerebellum and brain stem, 1 hand, 1 stomach) were selected for mycological evaluation at the Mycology Department from Fundacao Oswaldo Cruz.
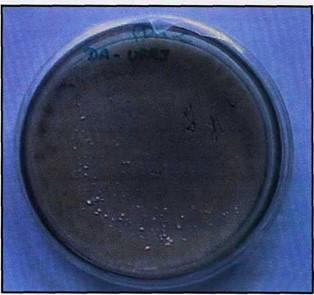
Figure 3. Culture media from surface fragments of plastinated specimens showing Aspergillus fumigatus growth (white spots).
In that Institution the specimens were slightly scraped and the fragments were sed in Petri dishes with Potato Dextrose Agar (PDA - Difco), which is a standard medium for isolation of a wide range of fungi (Moraes et al., 1998), and incubated at room temperature (+/- 28° C). During the first ten days, daily examinations were carried out in order to observe fungal growth (figure 3), followed by examinations every 3 days until the 21st day.
The isolated colonies were subcultured in Malt extract and Czapeck-dox (Difco) medium for identification. The microscopic characteristics produced by the species were studied using the technique of culture on slide (Rivalier and Seydel, 1932). The material was colored with 10% KOH for the representative with dark pigmentation and with Amann's Lactophenol with cotton blue for the hyaline isolated representatives and observed under a Nikon model Labophot light microscope. Species identification was made according to Pitt (1979, 1985), Raper and Fennell (1965), Samson (1979) and Klich and Pitt (1994).
Samples of deeper tissues from the specimens were also evaluated, as well as the Biodur S10 silicone rubber and the silicone hardeners Biodur S3 and S6.
Fungicide protocol
Contaminated plastinated specimens have undergone a fungicide protocol developed by us, which consists of the following steps:
The Plastinated Specimens Facility shelves have undergone a similar protocol, as follows:
Mycological analysis of the plastinated specimens after application of the fungicide protocol
The specimens selected for the first mycological analysis went through the fungicide protocol and after 2 months were once again evaluated by the Mycology Department from Fundagao Oswaldo Cruz.
The cultures of plastinated specimens superficial fragments showed significant growth of different fungi species (Table 1). The Biodur products and deep tissues analysis showed no fungal contamination. The specimens which had undergone our fungicide Protocol did not show fungi infestation anymore.
| PLASTINATED SPECIMENS | FUNGI SPECIES |
| Kidney, Cerebellum and Brain stem | Penicillium janthinellum |
| Abdominal sagital section, Stomach, Hand | Penicillium corylophilum |
| Muscles of rotator cuff | Aspergillus niger |
| Heart | Aspergillus flavus |
| Abdominal transverse section | Aspergillus fumigatus |
Based on the fact that the specimens had been properly fixed before being correctly plastinated, according to the S10 standard technique, as well as stored in a low humidity, clean environment and considering that the mycological analysis of the Biodur products showed no contamination, we can conclude that the sudden environment humidity increase was the cause of fungal infestation, since it is well-known that such environment predisposes to the development and growth of habitual ambient fungi.
Since the plastinated specimens have been free from contamination 2 months after the fungicide protocol application, we can affirm that such protocol was efficient in eradicating the contaminating fungi. Up to this date (10 months after the fungicide protocol application) we have not detected any kind of macroscopic fungi growth in our specimens. The products used in the protocol were choosen since they are widely cited in literature being efficient for all kind of surfaces and live tissues, like insect cuticles and plants, without causing any harm (Hakwsworth, 1977), besides they are cheap products, such as formalin, granular chlorine and alcohol, easily found in supermarkets as well as in Anatomy departments and easy to use and prepare in the laboratory routine.
All the contaminated plastinated specimens (more than 500) went through this fungicide sequence and none has been harmed (figures 4 and 5), except for some white spots that could be seen in a few specimens, due to chlorine deposition, easily removed by brushing.
Concerning the consequences of fungal infestation, the process proved to be superficial and left superficial white, black and brown spots in some plastinated specimens (figure 6). These spots could be the result of the probable use of plastinated specimens as a substratum for parasites development. The promptly use of the fungicide protocol may reduce the chances of such consequence.
For prevention, we suggest the use of devices that reduce environmental humidity in the plastinated specimens storing room, such as air conditioners, specially in tropical countries. The Plastinated Specimens Facility should present a good infra-structure in order to prevent leakage and inundations, conditions that predispose to fungal growth.
The isolated genera, Aspergillus and Penicillium, are ubiquotous fungi. They are found worldwide in the most different hosts and substrats (Raper and Fennel, 1965; Pitt, 1979). So we suspected that they would be isolated from the material that were exposed to the high humidity that the water overflow caused and the results obtained, confirmed our expectations.
In relation to the pathogenic capacity of the fungi species, we should focus on Aspergillus. Aspergillus fumigatus, Aspergillus flavus and Aspergillus niger can be responsible for allergic bronchopulmonary aspergillosis in immunocompetent hosts, especially in individuals with an increased responsiveness of trachea and bronchi to various stimuli (asthma), chronic sinusitis and colonization of preexisting pulmonary cavities (Aspergilloma) (Bennet, 1998; Hamill and Hollander, 1997). In immunodeficient people, however, the infection is considered severe since the patient can develop invasive aspergillosis (Lortholary et al., 1993). This disease has a mortality rate well above 50%. The Penicillium infection is not clinically important in immunocompetent hosts.
We strongly suggest the use of gloves and masks during the fungicide process.
Acknowledgment
This study was supported by the Fundacao Universitaria Jose Bonifacio.
The authors wish to thank Dr. Katia F. Rodrigues, from Department of Micology of Fundacao Oswaldo Cruz, for her help with this project as well as Dr. Ana Helena Pereira Correia for the English writing supervision.
Bennet J: Aspergilose. Harrison Medicina Interna. 14th Ed. Mc Graw Hill, Vol 1: pp 1238-1239, 1998.
Correia JAP, Prinz RAD, Freitas ECB, Pezzi LHA: Labelling and Storing Plastinated Specimens - An Experience from Universidade Federal do Rio de Janeiro. J Int Soc Plastination 13 (2): 17-20, 1998.
https://doi.org/10.56507/LTAD6864
Hamill RJ, Hollander H: Infectious Diseases: Mycotic. Current Medical Diagnosis & Treatment. 36th Ed. Connecticut, Appleton & Lange: pp 1363-1364, 1997.
Hawksworth DL: Mycologist's Handbook. 2nd Ed. CAB Press, London, 1977.
Klich MA, Pitt JI: A laboratory guide to common Aspergillus species and their teleomorphs. 2nd Ed. CSIRO, Australia, 1994.
Lortholary O, Meyohas MC, Dupont B, CadranelJ, Salmon-Ceron D, Peyramond D, Simonin D: Invasive aspergillosis in patients with acquired immunodeficency syndrome: Report of 33 cases. Am J Med 95: 177-180, 1993.
https://doi.org/10.1016/0002-9343(93)90258-Q
Moraes AML, JunqueiraACV, Giordano CM: Aspergilli from the digestive tract of Brazilian triatomids. Mycotaxon 66: 231-241, 1998.
Pitt JI: The genus Penicillium. Academic Press, Sidney, 1979. Pitt JI: A laboratory guide to common Penicillium species. Academic Press, Sidney, 1985.
Raper KB, Fennell DI: The genus Aspergillus. The Williams and Wilkins Co., Baltimore, 1965.
Rivalier E, Seydel S: Nouveau procede de culture sur lames gelosees applique a l'etude microscopique des champignons et des teignes. Ann Parasitol 10: 444-452, 1932.
https://doi.org/10.1051/parasite/1932105444
Samson RA: A compilation of the Aspergilli described since Studies in Micology 6: 1-119, 1979.
von Hagens G: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomisches Institut 1, Universitat Heidelberg, Heidelberg, Germany, 1985.