1 Plastination Laboratory, Department of Morphology, Biosciences Center, Federal University of Rio Grande do Norte, Rio Grande do Norte, Brazil
2 Multicampi School of Medical Sciences, Federal University of Rio Grande do Norte, Rio Grande do Norte, Brazil
This paper describes the implementation of a plastination laboratory at a public university in northeastern Brazil. We present the challenges related to the establishment and operationalization of the Plastination Laboratory at the Federal University of Rio Grande do Norte (LabPlast/UFRN) and the equipment and substances indispensable for the standard protocol of the plastination technique. We discuss the importance of using plastinated anatomical specimens for teaching human anatomy at UFRN as an alternative to complement the studies of health students. Finally, we also discuss the future perspectives of LabPlast/UFRN for research and extension activities at UFRN as a way to popularize and demystify human anatomy for Brazilian society.
LabPlast/UFRN; plastination; challenges; human anatomy teaching; education
Prof Aldo F. Souza, Plastination Laboratory, Department of Morphology, Biosciences Center, Federal University of Rio Grande do Norte, Rio Grande do Norte, RN, 59064-741, Brazil.
Tel. +55 84 996980973; E-mail: aldofs.neuro@gmail.com
![]()



Human anatomy is the core of many health courses. Knowing and identifying different structures of the human body are the fundamental principles of this academic subject. It brings many challenges ranging from the complexity related to nomenclature and identification of structures, to the topographical relationships of the organs. Beyond that, there are other educational challenges related to learning Anatomy, such as the use of different instruments, the applicability of techniques, and the methods for preserving cadavers, which are the main resources of this fascinating and historical science.
Due to its considerable popularity, different laboratories and science institutes worldwide have adopted Dr. Gunther von Hagens' plastination technique (Latorre et al., 2007; Pashaei, 2010; Durand et al., 2011; Șora et al., 2012; Șora, 2016). As Riederer (2014) points out, over the past 20 years, plastination has become an essential means of preserving organs for well-dissected specimens or body slices. This technique is based on replacing water and body lipids with a synthetic component (polyester, silicone, or epoxy) (Hayat et al., 2018) through a vacuum system.
The main challenge for the use of plastination in Brazil is related to the lack of investment, especially in public universities. The recent advent of a plastination laboratory at a public university in northeast Brazil has allowed many undergraduate students to understand the technique and its advantages. In this article, we discuss this implementation with emphasis on the challenges and opportunities of establishing this laboratory in a public Brazilian University. In addition, we also highlight the contribution to the formation of future health professionals and perspectives for research and extension activities, a strategy to popularize and demystify the knowledge of human Anatomy.
The Plastination Laboratory of the Federal University of Rio Grande do Norte (LabPlast/UFRN) was a challenging project with the first ideas initiated in 2009. Celcimar Câmara, Professor of Human Anatomy at the Department of Morphology at UFRN, conceived these ideas that were materialized ten years later with the indispensable participation of the Multicampi School of Medical Sciences at UFRN. The main motivation for the implementation of the LabPlast/UFRN was to serve as another academic space for the production of scientific knowledge, to complement the studies of health students, and to serve as another research unit within the university.
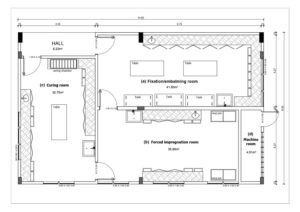
Figure 1. Floor plan of plastination laboratory of the Federal University of Rio Grande do Norte (LabPlast/UFRN): a) Fixation/embalming room, b) forced impregnation room, c) curing room, d) machine room
The LabPlast/UFRN is located on the UFRN main campus in the Biosciences Center in Natal, Brazil. With an area of 132 m² (Fig. 1), the laboratory was built according to the original specifications, and designed solely to develop the plastination technique. The laboratory comprises three procedural rooms (fixation/embalming, forced impregnation, and curing) and a machine room (Fig. 2).
The fixation/embalming room (Fig. 2b) is 41.50 m² in area and corresponds to the first procedural room. In this room, the anatomical specimens (whole bodies or body parts) are fixed with formaldehyde through an injector pump (Protanato®, Fig. 3a) or brought already fixed from the institution's own human anatomy laboratory located in front of the LabPlast/UFRN. In the fixation/embalming room, the specimens are also dissected and stored in plastic buckets before being dehydrated in acetone in the forced impregnation room.
Eventually, after dissection and conditioning of the specimens in a formaldehyde solution, we also adopted a protocol of washing the specimens in running water, followed by immersion in a mixture of ethanol (70%) and hydrogen peroxide (3%) in the proportion of 1:1 for four or five days to lighten darker specimens, and then cooling to 4 °C for 24 h before

Figure 2. Plastination laboratory of the Federal University of Rio Grande do Norte (LabPlast/UFRN): a) facade of the LabPlast/UFRN, b-d) procedural rooms of laboratory, b) fixation/embalming room, c) forced impregnation room, d) curing room, e) machine room
dehydration in acetone.
After complete fixation and dissection, the specimens are transferred to the forced impregnation room (Fig. 2c), where they are placed in containers containing acetone at -20 °C (Fig. 3c). There, the specimens are dehydrated using acetone as the standard solvent for the plastination technique. Customarily, we use a volume of acetone sufficient to cover 2 cm above the specimens. This first acetone bath is done at a lower concentration than acetone PA, usually between 90-95%, in order to avoid fast shrinkage of the specimens in a short time. Monitoring the acetone concentration is done using an acetonometer. A 500 ml sample of the solution is taken daily and placed in a graduated cylinder. We consider a reliable titration at a temperature of 20 °C.
After the first stabilization of acetone concentration, the specimens are immersed again in acetone solutions with increasing concentrations until they are finally dehydrated in acetone PA. The dehydration time varies according to the quantity and size of the specimens, as well as with the predominance of the type of biological tissue present in the specimens. Since we do not plastinate very large specimens, the dehydration time of the specimens varies from 15 to 21 days. We consider specimens completely dehydrated and ready to be impregnated when the acetone concentration reaches 98 or 99% purity.
After complete dehydration, the acetone-soaked specimens are transferred to the refrigerated container (Fig. 3d) containing the mixture of S10 silicone and S3 hardener in a 100:1 ratio. Initially, the specimens are arranged in a

Figure 3. LabPlast/UFRN equipment and materials: a) injection pump, b) S10 Biodur® silicone drum and dehydration freezer, c) top view of dehydration freezer and acetone conditioning containers, d) top view of impregnation freezer and impregnation chamber with S10B dyed silicone, e) pressure manometer, f) overview of the forced impregnation room duct system, g) front view of the vacuum pump, h) lateral view of the vacuum pump, i) curing chamber fan, j) curing chamber
stainless-steel basket and placed in the container. Twenty-four hours later, a vacuum pump (Fig. 3g,h) located in an adjacent room (machine room, Fig. 2e) is activated, starting the forced impregnation procedure. The impregnation is monitored daily by laboratory technicians who observe the speed and size of the impregnation bubbles that appear during the process. Vacuum adjustment is done daily on both the pump valve and the manometer needle valves (Fig. 3e). When the vacuum pump valve is fully open, the adjustment is only done at the needle valves inside the forced impregnation room. The impregnation time can vary depending on the type, size, or quantity of specimens. In our lab, we consider anatomical specimens to be fully impregnated when the pressure reaches at least 20 mbar.
When the pressure inside the system reaches 20 mbar and no impregnation bubbles appear, the vacuum pump is switched off and the impregnation chamber is depressurized. The glass cover of the impregnation chamber (Fig. 3d) is carefully removed and the basket with the specimens is suspended so that the first excess resin can be drained into the impregnation chamber. Immediately following this, the specimens are removed from the basket and placed on steel grids so that the second excess resin is drained from the specimens into plastic trays placed below the grids. At this point, we also used paper towels to facilitate the "cleaning" of the specimens, as well as their initial positioning.
About two hours after the specimens have been removed from the impregnation chamber, they are transferred to the third procedural room, the curing room (Fig. 2d). In the curing room, the final positioning of the specimens is performed. In this step, we use different objects, such as pins, string, hooks and fasteners, which allow us to position the specimen as we wish. As the main purpose of the LabPlast/UFRN plastinated specimens is to complement human anatomy studies, we strive to always leave them in the standard anatomical position. Once properly positioned, the specimens are transferred to the curing chamber (Fig. 3j), being placed on stainless steel grids. A ventilator (Fig. 3i) coupled to the curing chamber accelerates the process of volatilization of S6 silicone hardener, in Erlenmeyer (conical) flasks, which will penetrate the tissues of the specimen, making it more rigid and durable. As a standard protocol at this stage, we do two curing cycles, twice a day, in different shifts, for two or three days.
After the final curing cycle, when all residual resin is gone and we observe that the specimen is dry, we remove it from the curing chamber and let it rest on paper towels for two weeks. After this, the plastinated anatomical specimens are ready for use by professors and students.
Plastination is a remarkable technique, but it requires considerable care and safety. From the organization of the physical space to the handling of substances used in the technique, attention to safety aspects of the work is a crucial factor to minimize the occurrence of damage to the health of laboratory users, as well as damage to the building's infrastructure.

Figure 4. Work safety equipment at LabPlast/UFRN: a) external exhaustion system: thick white arrows indicate where the vapors are removed from the internal environment of the forced impregnation room, black dashed arrows indicate the flow of vapors through the ducting until they are released into the external environment, b) internal exhaust system with explosion-proof plug inserted into the outlet (arrow), c) type B/C fire extinguisher, d) eyewash station
Because acetone is a highly volatile and flammable substance, all rooms of LabPlast/UFRN are equipped with type B and type C fire extinguishers (Fig. 4c). The laboratory is equipped with standard fluorescent lamps and explosion-proof plugs (Fig. 4b). Furthermore, no common electrical outlets are present in the impregnation room. During activities at LabPlast/UFRN, the air conditioners remain on from the beginning (6:30 am) until the end of the day (5:00 pm), keeping the room at a temperature of 19 °C. As with the other UFRN buildings, LabPlast/UFRN is also grounded (electrically earthed).
Mostly, we produce cold temperature plastinated specimens. Thus, the acetone is stored in two rectangular stainless-steel containers with a lid (124 x 24 x 60 cm) inside freezers (Fig. 3c). Each dehydration container has the capacity to store 170 liters of acetone, but we never use that volume. In fact, we often dehydrate specimens in buckets with a much smaller volume capacity, in order to avoid using large volumes of acetone. The acetone drums are stored in a separate location in the Morphology Department at UFRN, in a shaded, locked, but well-ventilated and airy environment. Both the acetone and the resin that will no longer be reused are properly identified and rigorously stored in specific drums and later delivered to the responsible professionals in the environment sector, who will arrange for the disposal of these materials in an appropriate place.
We do not use explosion-proof freezers. Normally, BIODUR® freezers are adapted to the special needs of plastination. The bottom is reinforced by a metal plate, and the refrigeration unit is removed and placed together with the temperature control unit in a separate case, together with vacuum pumps in the machine room (Fig. 2e), being ventilated by natural ventilation. Thus, potential ignition sources are kept away from the actual working area in which flammable solvents will be used. During the impregnation, when the vacuum pump is activated, we also turn on a mechanical fan which enhances the natural ventilation, decreasing the internal temperature of the machine room. Additionally, in the fixation/embalming room and the forced impregnation room, the LabPlast/UFRN is equipped with external (Fig. 4a) and internal (Fig. 4b) exhaust systems, while in the curing room there is only an internal exhaust system, which removes acetone vapors and other substances harmful to human health from the internal environment of the rooms.
Finally, the technicians of the LabPlast/UFRN, responsible for the production of all plastinated specimens, wear personal protective equipment rigorously. Full-face shields and rubber gloves are always used when handling acetone, S10 silicone, and S3 or S6 hardener. An eyewash station (Fig. 4d) in the curing room also contributes to the collective safety of these professionals.
All procedures related to the plastination technique performed at LabPlast/UFRN are highly professional, ethical, and respectful regarding the cadaveric anatomical specimens.

Figure 5. Examples of anatomical specimens plastinated in the Plastination Laboratory of the Federal University of Rio Grande do Norte (LabPlast/UFRN): a) fetus (anterior view) showing thoracic and abdominal viscera, b) fetus (posterior view) showing segments of the spine, spinal cord, kidney, adrenal gland, and lung, c) medial view of hemi-head and neck showing cranial cavity, dura mater, oral cavity, nasal cavity, pharynx, esophagus, larynx, trachea, and cervical part of the spine, d) esophagus, stomach, duodenum, and pancreas, e) testis and spermatic cord, f) knee joint, g) heart and great vessels, h) urinary bladder and prostate sectioned showing neoplastic formation (white arrow), i) accessory glands of the male genital system (seminal vesicles, prostate, and bulbourethral glands), parts of the vas deferens and root of the penis
The LabPlast/UFRN has produced more than 160 anatomical specimens since its inauguration in 2019. The quality of the plastinated specimens surprises undergraduate students. The initial perception about the use of the plastinated specimens in Anatomy classes in UFRN is that the students seem to be satisfied and motivated to learn with these specimens. In some cases, we realized they would prefer to study these specimens rather than those fixed with formaldehyde. It is possible to “eternalize” anatomical variations or pathological conditions for didactic purposes through the plastination technique. For example, we can plastinate a specimen with a prostatic neoplasm (Fig. 5h). Some of these anatomical specimens produced at LabPlast/UFRN are illustrated in Figure 5.
We also are conducting some research in the laboratory. One of them is the investigation of different natural or artificial dyes for use in the plastination technique. A pilot experiment carried out in our laboratory has shown that some pigments easily found in nature, or sold in supermarkets, adhered to the surfaces of chicken hearts even after being dehydrated in acetone and cold impregnated (Fig. 6).
In parallel to the production line of plastinated anatomical specimens in the LabPlast/UFRN, we are producing an image database of plastinated specimens and an atlas, using the lab's samples to assist the students' learning process during the human anatomy course. These steps help to comprehend the importance of using the plastination technique across higher education curricula in the health sciences field.
The LabPlast/UFRN was designed to implement an innovative teaching methodology in Human Anatomy at a Public University in Northeastern Brazil in 2010. Over the last ten years, several processes have been formatted in order to make its implementation feasible. The architectural project was designed to meet all the safety conditions required for a laboratory that uses flammable liquids such as acetone. Several steps of the project were guided by the German company BIODUR through on-site technical support in 2019. The plastination laboratory at UFRN currently plays a strategic role in the context of consolidating the teaching of Human Anatomy, not only on the central campus of the University, but also in health-related courses in cities far from the capital of the state of Rio Grande do Norte.
Undoubtedly, many challenges have emerged since the foundation of LabPlast/UFRN until its operationalization. The initial reluctance of the idea by some professors, the delay in the approval of the project for the construction of the laboratory, the acquisition of equipment and substances from BIODUR for the plastination technique, and the transfer of these materials to the state of Rio Grande do Norte, in northeastern Brazil, were some of these obstacles.
Although the laboratory was ready for use and production of the plastinates, it lacked the technical operational staff to manage and produce these specimens, and to control the use of the consumable supplies. Fortunately, one year after LabPlast's inauguration, two technicians were hired by UFRN and assigned exclusively to LabPlast/UFRN to learn and conduct the plastination technique with autonomy and independence.
One challenge we usually face is the acquisition of the substances for the plastination technique. As we purchase the supplies from the German company BIODUR, the procurement process is often very costly and time consuming, especially in relation to the release of the products by the Brazilian customs control. Consequently, this can make it difficult to produce some specimens on time.
The anatomy laboratories at UFRN receive about 800 students per year under normal conditions. They are from various health courses such as medicine, dentistry, nursing, physiotherapy, speech therapy, among others. With the arrival of the COVID-19 pandemic caused by the new coronavirus SARS-CoV-2 also came the challenges related to online classes. However, the possibility of using the plastinated samples during the online human anatomy classes was a positive point.
As face-to-face classes at UFRN were interrupted by the worsening of the COVID-19 pandemic, many professors needed to modify their way of teaching human anatomy, adapting to this new scenario of social distance and online classes. Thus, even remotely, some professors used the plastinated anatomical specimens and their associated pathologies or anatomical variations to expose the organic structures to the students, thereby facilitating the teaching and learning process in the human anatomy module. Interestingly, still during the pandemic, with the staggered and controlled return of classes at UFRN, many students used plastinated specimens from the LabPlast/UFRN to study human anatomy, reaffirming the importance of this laboratory at UFRN.
In pandemic times, re-establishing and rethinking new ways of learning is fundamental to the teaching of anatomy. The technique of plastination, invented by Gunther von Hagens in 1977, offers the opportunity for scientists to produce durable preparations in their own labs (von Hagens et al., 1987; Schill, 2018; Henry et al., 2019), which can contribute positively to the learning of this academic subject.
Another positive outcome in using the plastination technique is its academic applicability at university campuses further away from large urban centers. For example, some UFRN campuses in the countryside cities across the state still use synthetic anatomical models to aid studies in human anatomy. Plastination brings with it this innovative perspective of studying real organs, which, despite their rigid and inflexible structure after the procedure, allows students to handle the specimens with their accurate anatomical and topographical relationships.
A considerable advantage observed in the plastination technique is the quality and durability of the anatomical specimens. It is possible to produce anatomical specimens which are odorless, free of moisture, and with a realistic appearance (Zerlotini et al., 2020). It is an ideal method for the long-term preservation of tissues, body parts, or whole bodies (Riederer, 2014).
Another important point to be highlighted alluding to anatomy classes, is the use of substances to preserve the cadavers. A common practice amongst Brazilian public universities, like other universities worldwide, is the use of formaldehyde on donated cadavers. This chemical substance allows the organic material to last longer, avoiding putrefaction, and can be used several times. Nevertheless, the constant handling of these anatomical parts reduces their useful life span. Professors and students are potentially at risk by being in direct contact with this substance for a prolonged time. In this case, plastination promotes a double advantage. Firstly, it ensures the durability of the anatomical specimens, and secondly, it considerably reduces the exposure of users to these substances.
Regarding the applicability and efficacy of the use of plastinates in learning of human anatomy classes, while only a few relevant studies have been undertaken thus, they show promising results, contrasting learning human anatomy through plastinated specimens, compared to non-plastinated specimens.
Through a cross-sectional study using questionnaires answered by 280 students of undergraduate medical and anatomy, Azu et al. (2012) showed that 94.29% rated plastinated prosections as a valuable resource for their anatomical learning and would want to learn about the technique. But, 78% said it should be used alongside other cadaveric materials. Paradoxically, in a subsequent paper, this same research group showed that the awareness and use of plastinated specimens as an additional learning resource in anatomy remains very low (Azu et al., 2021). The main results of this study that support the authors' conclusion is due to the low percentage of respondents about the motivation to learn the plastination technique, the replacement of cadavers by plastinated specimens, if plastinates were better than cadavers, and preference to be examined using plastinates (Azu et al., 2021).
Latorre et al. (2016) provided interesting results regarding learning human anatomy using plastinated specimens with students at Cambridge University, UK. The study showed that 97.7% of all students thought that the plastinates helped them understand and learn anatomy. In addition, all the students surveyed recommended the use of plastinated specimens in the future (Latorre et al., 2016). Corroborating the above study, research conducted by Bayko et al. (2018) investigated the views and awareness of medical students who receive education with anatomic plastinated cadavers. The results of this study showed that most students (60.2%) believed that the anatomy education provided with plastinated cadavers had a positive effect on their anatomical knowledge (Bayko et al., 2018).
A study of dental students and residents compared students' opinions and performance in learning anatomy through cadavers or plastinated specimens (Nguyen et al., 2019). The results showed that more than half of students in all cohorts believed that plastinated prosections can effectively replace the need for dissection. Moreover, the shift in learning anatomy from cadavers to plastinated specimens increased student satisfaction with anatomy instruction and improved student performance in the course (Nguyen et al., 2019).
A cross-sectional, observational study conducted by Brazilian researchers investigated the preference of human anatomy students from the dentistry, nursing and physiotherapy courses for the use of plastinated material compared to traditional models. The study showed that most students preferred to study plastinated anatomical specimens colored (64.2%) or uncolored (21.6%) (Pereira et al., 2013). Taken together, these studies signal a positive trend toward the increase and use of plastinated specimens. However, Chytas et al. (2019) reinforces that the importance of using plastination in teaching anatomy needs to be further investigated about its educational effectiveness (Chytas et al., 2019). Thus, to prove the effectiveness of using the plastination technique in the health curriculum, more comparative studies with significant results will be needed (Chytas et al., 2019).
On the other hand, the fact that there are few comparative studies on the opinion and/or effectiveness of use of the plastination technique in undergraduate students is perhaps evidenced by the low number of institutions that have appropriate spaces for the development of the technique. In Brazil, for example, there are few higher education institutions that have a specific place to produce plastinated anatomical specimens. Many of them are adapted due to the high cost of establishing and operating a specific laboratory for plastination (Zerlotini et al. 2020).
There are many different anatomical techniques used to preserve the structures of living beings. These techniques are crucial because they provide the maintenance and viability of human and animal anatomy to be used by students and professors of courses in the health and biological sciences at the undergraduate or graduate level. Moreover, these items could be applied for teaching, researching, or even as a traveling scientific museum exhibit. Dr. Gunther von Hagens' plastination technique has changed the preservation of biological structures and influenced people's perception of ethical issues behind the technique (Jones, 2002; Bin et al., 2016).
We have many perspectives for the future regarding LabPlast/UFRN. One of them is the production of the first entirely plastinated cadaver in the northeast of Brazil. Moreover, we are also developing the idea of producing animal specimens for teaching in the academic discipline of Comparative Anatomy of Vertebrates to contribute to the qualification of future biologists and biology teachers.
In addition, a recent research project involving LabPlast/UFRN and the Multicampi School of Medical Sciences (EMCM)/UFRN was approved, and we are working steadily to obtain the first results on the impact of different teaching methodologies in human anatomy for students of health courses at UFRN. We are certain that this project will contribute substantially to understanding the potential of plastination as a complementary tool, not only for teaching human anatomy, which is a reality in many higher education institutions around the world, but also its implications for university research and extension activities. Exhibitions of plastinated specimens in collaboration with UFRN’s Museum of Morphological Sciences are also actions designed to socialize and demystify Human Anatomy for the population.
In conclusion, the idea of building a laboratory specifically to develop the plastination technique in a state in the northeast of Brazil was a milestone that took at least ten years to be realized. Obviously, several challenges and obstacles were encountered along the way, but with the collaboration of several professors committed to the same goal, it was possible to achieve success. We believe that the positives have outweighed the negatives. This article is not just a technical article about the establishment of a laboratory. It is a way we found to socialize the LabPlast/UFRN for the entire scientific community that recognizes the importance of plastinated specimens for teaching and learning in Human Anatomy, this fascinating science.
Azu OO, Naidu ECS, Trinity M, Naidu JS. 2021: The use of plastinated specimens in anatomical education in The University of Kwazulu-Natal: need for advocacy. J Plast 33(1):4-12.
https://doi.org/10.56507/EICY3880
Azu OO, Peter AI, Etuknwa BT, Ekandem GJ. 2012: The awareness of medical students in Nigerian Universities about the use of plastinated specimens for anatomical studies. Maced J Med Sci 5:5-9.
https://doi.org/10.3889/MJMS.1857-5773.2011.0202
Bayko S, Yarkan IS, Çetkin M, Kutoglu T. 2018: Views of medical students on anatomy education supported by plastinated cadavers. Anatomy 12(2):90-96
https://doi.org/10.2399/ana.18.043
Bin P, Conti A, Buccelli C, Addeo G, Capasso E, Piras M. 2016: Plastination: ethical and medico-legal considerations. Open Med (Wars) 11:584-586.
https://doi.org/10.1515/med-2016-0095
Chytas D, Piagkou M, Johnson EO, Tsakotos G, Mazarakis A., Babis GC, Nikolaou VS, Kaseta M, Natsis K. 2019: Outcomes of the use of plastination in anatomy education: current evidence. Surg Radiol Anat 41:1181-1186.
https://doi.org/10.1007/s00276-019-02270-3
Durand M, Pourchez J, Louis B, Pouget JF, Isabey D, Coste A, Prades JM, Rusch P, Cottier M. 2011: Plastinated nasal model: a new concept of anatomically realistic cast. Rhinology 49:30-36.
https://doi.org/10.4193/Rhino09.187
Hayat K, Qureshi AS, Rehan S, Rehman T. 2018: Plastination - an innovative preservative technique in anatomy. Trends Anat Physiol 1:1-5.
https://doi.org/10.24966/TAP-7752/100003
Henry RW, von Hagens G, Seamans G. 2019: Cold temperature/Biodur®/S10/von Hagens'-Silicone plastination technique. Anat Histol Embryol 48:532-538.
https://doi.org/10.1111/ahe.12472
Jones DG. 2002: Re-inventing anatomy: the impact of plastination on how we see the human body. Clin Anat 15:436-40.
https://doi.org/10.1002/ca.10040
Latorre RM, Bainbridge D, Tavernor A, Albors OL. 2016: Plastination in anatomy learning: an experience at Cambridge University. J Vet Med Educ 43:226-334.
https://doi.org/10.3138/jvme.0715-113R1
Latorre RM, García-Sanz MP, Moreno M, Hernández F, Gil F, López O, Ayala MD, Ramírez G, Vázquez JM, Arencibia A, Henry RW. 2007: How useful is plastination in learning anatomy? J Vet Med Educ 34:172-176.
https://doi.org/10.3138/jvme.34.2.172
Nguyen VH, Pham PT, Joo K, Jeter CB. 2019: Dental students' and residents' opinions and performance of anatomy learning via cadavers or plastinated specimens. J Plast 31(1): 6-13.
https://doi.org/10.56507/KMZL8564
Pashaei S. 2010: A brief review on the history, methods, and applications of plastination. Int J Morphol 28:1075-1079.
https://doi.org/10.4067/S0717-95022010000400014
Pereira KF, Oda JY, Silva IN, Sant'ana HGF, Saldanha Filho AJM, Barros HP. 2013: Use of material plastinated detriment to traditional models: verification of predilection students of human anatomy. Arq Ciênc Saúde UNIPAR 17:105-108.
Pereira KF, Oda JY, da Silva IN, Sant'ana HG, Saldanha Filho AD, Barros HP. 2013: Utilização de material plastinado em detrimento aos modelos tradicionais: verificação da predileção de alunos de anatomia humana [Use of material plastinated detriment to traditional models: verification of predilection students of human anatomy]. Arquivos de Ciências da Saúde da UNIPAR 17(2):123-6. (In Portugese)
Riederer BM. 2014: Plastination and its importance in teaching anatomy. Critical points for long-term preservation of human tissue. J Anat 224:309-315.
https://doi.org/10.1111/joa.12056
Schill VK. 2018: General issues of safety in plastination. J Plast 30 (1): 27-36.
https://doi.org/10.56507/NJCY9228
Șora, MC. 2016: The general protocol for the S10 technique. Res Clin Med 1:14-18.
Șora MC, Jilavu R, Matusz P. 2012: Computer aided three-dimensional reconstruction and modeling of the pelvis, by using plastinated cross sections, as a powerful tool for morphological investigations. Surg Radiol Anat 34:731-736.
https://doi.org/10.1007/s00276-011-0862-2
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175:411-421.
https://doi.org/10.1007/BF00309677
Zerlotini MF, Paula TAR, Ramos ML, Silva FFR, Santana ML, Figueira MP, Silva LC, Silva VHD, Bustamante LRC. 2020: Establishment and operationalization of a low-budget plastination laboratory in the Veterinary Morphology Section of the Federal University of Viçosa, Minas Gerais - Brazil. J Plast 32(1): 8-17.
https://doi.org/10.56507/RKZU4777