Departement de chimie-biologie, Universite du Quebec a Trois-Rivieres, Trois-Rivieres, Quebec, Canada
The aim of this project was to provide students with plastinated specimens showing the three-dimensional features and contents of the human brain ventricular cavities. To fill the cavities, six cannulas were introduced through the cerebral cortex into the horns of both lateral ventricles, and a seventh one between the pons and the cerebellum into the fourth ventricle. Ventricles were filled either with 10% gelatin mixed with acrylic stain, or with a mixture of S10/S3/S6/S2 dyed with Biodur color paste. The brains were dissected to expose all ventricles and their communications (interventricular foramina, cerebral aqueduct), before being plastinated according to the standard S10 procedure. Gelatin and silicone casts were compared to each other.
Presented in part at the 6th interim conference on plastination, Rochester, NY, USA, July 11-16,1999.
Human brain, Ventricular cavities, Silicone cast, Gelatine cast
Gilles Grondin, Departement de chimie-biologie, University du Quebec a Trois-Rivieres, C.P. 500, Trois-Rivieres, Quebec, Canada G9A 5H7. Telephone:819 376 5053 / Fax:819 376 5084. Email: Gilles_Grondin@uqtr.uquebec.ca
![]()



Though crudely depicted by the Italian Guido da Vigevano as far back as in 1345 (Olry, 1997), the famous Leonardo da Vinci has to be regarded as one of the very first anatomists to have attempted to make an accurate anatomical description of the ventricular system of the brain. In that regard, he performed wax injection of the brain ventricles in the late fifteenth or early sixteenth century (Huard, 1968). As usual at that time, he did not use human but ovine brains. In the following decades, anatomical descriptions of the brain ventricles became more accurate (figure 1). However, the technique used by Leonardo was so perfected that it took about two centuries before another anatomist, Daniel Duncan (1678), could obtain similar results as his renowned predecessor.
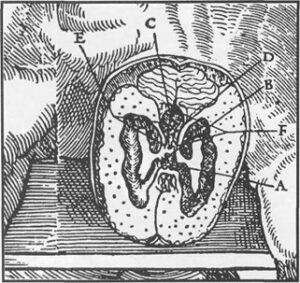
Figure 1. Engraved plate showing the morphology of the human brain ventricles, taken from the 1545 edition of Charles Estienne's De Dissectione Partium Corporis Humani (detail).
Nowadays, most of anatomical illustrations of the brain superpose an outline of the ventricular cavities on the brain: ventricles are seen showing through. However, this kind of illustration is not enough to understand their three- dimensional features and relationships. Ventricular casts which were previously produced by injection of various substances were always removed from the brain before being displayed. Our study provided students with specimens showing injected ventricular system of the brain in situ.
Preparation of silicone as well as epoxy casts of various cavities has been described previously (Henry, 1992a, 1992b; Pretorius et al., 1995; Henry et al., 1998). Colored gelatin has also been suggested to fill blood vessels or the gallbladder (Olry and von Hagens, 1992) before plastination. It was therefore decided to produce silicone and gelatin casts of the ventricular system of the brain in order to compare the results obtained with these two media.
The brains used in this study were removed from bodies of the dissection room. These bodies had been perfused via the right common carotid artery with our standard embalming mixture: ethanol (70%), glycerol (20%), formalin (4%), dettol (3%), phenol (2%), and sodium acetate (1 %). After injection, they were kept in a cold room for 8 to 12 months before being dissected. About 4 months after the opening of the dissection course, the brains were removed and kept in 10% formalin.
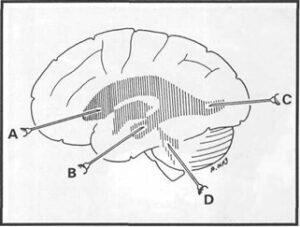
Figure 2. Drawing showing injection sites: anterior horn of the lateral ventricles (A), inferior horn (B), posterior horn
(C) and fourth ventricle (D).
Seven cannulas (14G x 57 mm) were carefully introduced into the brain cavities: six for the inferior, anterior, and posterior cornua of both lateral ventricles, and the last one for the fourth ventricle (figure 2). The first six cannulas were introduced in the depth of sulci, so that the injection sites are not visible on the plastinated specimen. The last cannula was introduced into the fourth ventricle between the posterior aspect of the medulla oblongata and the cerebellum. To ascertain that cannulas were in good position, water was injected into the ventricles with the expectation that water would flow from any of the properly positioned cannulas. However, leaks were observed through the floor of the third ventricle. To avoid leaking of the injection media, the brains were embedded in 10% gelatin.
Injection of the casting substance (gelatin or silicone) was then performed via one cannula. As soon as the injection medium could be observed leaking through another cannula, it was clamped and the same procedure went on until all cannulas were clamped. Ventricles of the first brain were filled with a solution of 10% gelatin dyed with acrylic artist color. After injection, the specimen was placed in water in the cold room for 48 hours before being dissected. The second specimen was injected with silicone. A Biodur S10/S3 mixture diluted with 30% methyl-ethyl-ketone and colored with Biodur color paste (AC50 and AC52) was used. Biodur S2 and S6 (0.3% of each) were added to the mixture just before the injection. The specimen was then stored in water in the cold room until the mixture was cured. Complete curing was estimated by the observation of the silicone mixture that remained in the preparation beaker.
Dissection was started immediately after curing of the injection mixture. The gelatin injected specimen was stored in 5% formalin for one week before being dehydrated. Specimens were wrapped in cheese cloth, rinsed in running tap water for 16 hours, and dehydrated according to the freeze substitution method (von Hagens et al., 1987). Impregnation was performed with Biodur S10 at room temperature over a 6 days period (table 1), according to the method described by Ripani et al. (1994). After the impregnation, the specimens were placed on a grid for 48 hours to permit drainage of the excess silicone. Dissection was completed at this stage. Specimens were finally fast cured with Biodur S6 over 5 days.
| Day / hours | Pressure |
| Day 1 8:30 17:00 |
Atmospheric 33 cm |
| Day 2 8:30 17:00 | 29 cm 15 cm |
| Day 3 8:30 17:00 |
13 cm 11 cm |
| Day 4 8:30 17:00 |
10 cm 9 cm |
| Day 5 8:30 17.00 |
8 cm 7 cm |
| Day 6 8:30 | Atmospheric |
The specimen injected with gelatin (figure 3) was of poor quality. The cast was brittle, and shrinkage of the gelatin gave rise to cracks that appeared during the plastination process. Moreover, brain tissue tended to stick to the cast, making the final dissection more difficult.
The specimen injected with silicone (figures 4-6) was unquestionably the most successful. The cast was flexible, and no shrinkage could be observed. Final dissection was much easier as the cast separated easily from the surrounding nervous tissue. All the ventricular system of the brain has been well injected: lateral ventricles (figures 4 - 5), third ventricle and interventricular foramina (figure 5), cerebral aqueduct and fourth ventricle (figure 6). Though perfectly injected, the cerebral aqueduct is not clearly visible because of its small calibre. None of the two specimens permitted the view of the choroid plexus.
The aim of this study was to provide students with ventricular casts in situ. We compared two different substances, gelatin and silicone. The latter proved obviously to be the best medium to be used for casts of the ventricles. However, the quality of the specimen will still have to be increased in the next future. We will try to find the ideal color for the silicone cast, so that the choroid plexus remains more visible showing through.
It is well known that S10 can last for a very long time at room temperature. However, as soon as the S3 is added to it, a reaction is initiated that will make the mixture become more and more viscous. This reaction can be slowed down or inhibited by cold temperature. The recommended storage temperature of the mixture is -70°C (Biodur, 1994) but most of the plastinators store it between -20°C and -25°C. For these reasons the percentage of methyl-ethyl-ketone used in this study (30%) might have to be modified (10 to 30%), depending on the viscosity of the S10/S3 mixture to be used. One should calculate the percentage to be added in order to obtain a mixture that will be easy to inject.
Before embedding into gelatin, excess formalin must be removed from the brains by thoroughly rinsing. Otherwise, fixation of the gelatin will make it hard and it will stick on the nervous tissue. This may explain why we had difficulties to perform the dissection of this specimen. Formalin could p have remained in the cavity.
Gelatin being a protein, we decided to post-fix the specimen after dissection to make sure that the cast was accurately fixed before being plastinated. As it was described before to be useful for filling cavities (Olry and von Hagens, 1992) and as we use it regularly to fill the gallbladders before plastination with S10, we expected it to make a good cast. We also thought that it could be a good choice for laboratories using only silicone for plastination without having access to small quantities of epoxy or other casting material because it is very cheap and easily available.
Biodur Company: Biodur™ Products, Polymers, Auxiliaries, and Equipment for Plastination, Catalog & Price List. Heidelberg, Germany, p 35, 1994.
Duncan D: Explication nouvelle et mecanique des actions animates, ou il est traite, de la fonction de l'ame, avec une methode facile pour dgmontrer toutes les parties du cerveau sans couper sa substance, et un discours sur sa formation. Paris: Jean d'Houry, 1678.
Estienne C: De dissectione partium corporis humani. Paris:Simon de Colines, 1545.
Henry RW: Silicone Tracheobronchial Casts. J Int Soc Plastination 6 (1): 38-40, 1992a.
https://doi.org/10.56507/LOVB7516
Henry RW: Silicone Pulmonary Vascular Casts with Attached Tracheobronchial Casts. J Int Soc Plastination 6 (1): 41- 44, 1992b.
https://doi.org/10.56507/MKMC2434
Henry RW, Daniel GB, Reed RB: Silicone Castings of the Chambers of the Heart and the Great Vessels. J Int Soc Plastination 13 (1): 17-19, 1998.
https://doi.org/10.56507/AWGT3303
Huard P: L6onard de Vinci. Dessins anatomiques (anatomie artistique, descriptive et fonctionnelle). Paris: Roger Dacosta, 1968.
Olry R: Medieval Neuroanatomy: the Text of Mondino dei Luzzi and the Plates of Guido da Vigevano. J Hist Neurosci 6 (2): 113-123,1997.
https://doi.org/10.1080/09647049709525696
Olry R, von Hagens G: Dissection and Plastination of Intestinal « en bloc » Specimens. 6th International Conference on Plastination, Kingston, Ontario, Canada, 1992.
Pretorius WF, Geyer HJ: The Use of E20 Red Resin for Casting Anatomical Cavities. J Int Soc Plastination 9 (1): 37,1995.
https://doi.org/10.56507/JQTR9706
Ripani M, Bassi A, Perracchio L, Panebianco V, Perez M, Boccia ML, Marinozzi G: Monitoring and Enhancement of Fixation, Dehydration, Forced Impregnation and Cure in the Standard S10 Technique. J Int Soc Plastination 8 (1): 3-5,1994.
https://doi.org/10.56507/ERSG2637
von Hagens G, Tiedemann K, Kriz W: The current potential of plastination. Anat Embryol 175 (4): 411-421,1987.
https://doi.org/10.1007/BF00309677