Section of Anatomy, Department of Morphology, Genetics and Aquatic Biology, Norwegian School of Veterinary Science, Oslo, Norway
This short technical note describes a method of filling blood and other vessels with epoxy using a 100ml syringe barrel with a catheter tip. The plunger of the syringe is replaced by a rubber stopper connected to a source of compressed air. The stopper is held in place by a simple home-made jig. After the syringe is filled with epoxy and the system assembled and switched on, epoxy may be pumped into the specimen for any chosen pressure and time combination without a person needing to be in attendance.
Polymer E20, Equipment, Cast, Compressed Air
David J. Griffiths, Section of Anatomy, Department of Morphology, Genetics and Aquatic Biology, Norwegian School of Veterinary Science, Post Office Box 8146 DEP, N-0033 Oslo, Norway Tel: 47 22964545 / Fax: 47 22964764 E-mail: david.griffiths@gveths.no
![]()



Coloured epoxies, such as Biodur™ E20 (Biodur, Rathausstrasse 18, 69126 Heidelberg, Germany), are excellent for casting blood vessels, bile ducts, the renal pelvis, and numerous other body cavities (Fromm et al., 1991; Riepertinger and Heuckendorf, 1993; Pretorius and Geyer, 1995). For injecting long and narrow vessel systems, the somewhat viscous nature of epoxy means that either very small syringes need be used repeatedly (Pretorius and Geyer, 1995) or the epoxy thinned with methyl-ethyl ketone (Fromm et al., 1991). The former method is labour-intensive and often messy, while evaporation of the latter reduces the strength of the cast and also its suitability for subsequent scanning electron microscopy. The following article describes a simple technique utilising compressed air to blow small or large volumes of undiluted epoxy into vessels in specimens.
A 100ml plastic syringe (Codan Medical ApS, Rodby, Denmark) with a catheter tip ideal for attaching a flexible silicon or rubber hose was used. The plunger was removed and a suitable rubber stopper was placed into the syringe barrel. A hole was drilled through the stopper and into this hole a 10mm diameter copper pipe was fed. The pipe was connected via a flexible hose to a supply of compressed air. AT-piece (not pictured) was inserted into the air supply close to the syringe, and out of the side tube a separate rubber hose led to a simple U-shaped water manometer set up on a wall of the laboratory. In this way the air pressure in the syringe could be measured with precision.
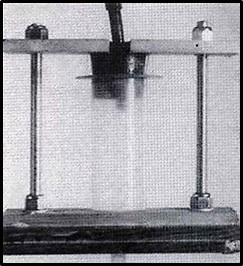
Figure 1. Syringe, stopper and stand. The feeder line for air enters the top of the stopper, while the outlet to the specimen is seen at the base. The top-plate of the stand holds the stopper in place and is tightened with the nuts until the seal is air-tight.
The jig used (figure 1) consisted of a wooden base, two lengths of threaded rod topped with wingnuts, and a wooden plate that moved snugly along the threaded rod. A hole in the baseplate and a corresponding slot in the upper plate held the syringe and its rubber stopper with protruding air-line. The specimen to be injected was fresh and unfixed, and was perfused overnight with tap water at a pressure of approximately 120cm water. When connecting the jig, the outlet hose from the syringe was tied into the desired artery using ligation with a braided linen thread of diameter some 0.5mm, and the syringe filled with the desired quantity of epoxy. The stopper was inserted into the syringe, then forced down using the plate and wingnuts, until there was no leakage of air. The compressed air supply was connected and the pressure adjusted according to the manometer. It is not necessary to clamp the lower hose to the syringe, for if its diameter is chosen correctly it stays in place on the tip by its own tightness. Although the volume of air in the system was reduced when feasible, no attempt was made to remove all air prior to injection. It was assumed that the air would be pushed ahead of the advancing epoxy and eventually be forced out of the arteries into the tissues.
At the very least this simple system provides a method of avoiding repeated and tedious pressing on a small syringe when injecting viscous solutions. However, since taking it into use other advantages have become apparent.
The first of these advantages is predictability, in that you know the exact pressure exerted on the epoxy. Since this pressure is constant for the entire injection time, the time- pressure combination can be correlated to the result obtained in the specimen. Once the most effective combination is determined, this can be used repeatedly and exactly, provided of course that the diameters and lengths of the hoses leading into the animal or specimen are not altered. Since the pressure loss along a length of hose (assuming laminar flow and all other factors constant) is proportional to the fourth power of the radius (Stephenson, 1969), halving the radius (for example) will produce a sixteen-fold increase in the resistance to flow and a great increase in the air pressure necessary in the syringe. It follows that the hose from the syringe into the specimen should be as short and as wide as possible.
The other advantage concerns time. With this method the epoxy can be pressed into the specimen right until it starts to harden, which in my experience with Biodur E20 is about 40 minutes. No time is lost in repeated disconnecting and refilling syringes, and no air is introduced unnecessarily into the system. The likelihood of vessel rupture is reduced.
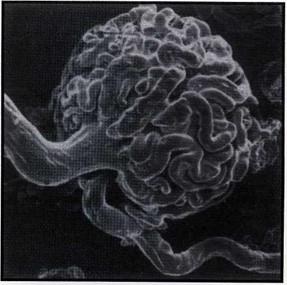
Figure 2. Injection of a canine renal artery using a pressure of 1.5m of water resulted in the epoxy traversing the glomerulus and entering the efferent arteriole, giving a very satisfactory cast.
This technique has to date been used for five specimens - two canine kidney pairs (via the aorta), the coronary arteries of a canine heart (via the aorta) and a dried porcine lung (via the truncus pulmonaris). Unless otherwise stated, Biodur E20/ E12 was used with a syringe pressure of 1.5m of water for a timespan of some 20 minutes. For the kidneys, some 70 ml of epoxy were placed in the syringe and some 40 ml pumped into the organs. Figure 2 shows the result of one of these attempts. The epoxy has traversed the glomerulus and entered the afferent arterioles.
For the heart, 70 ml of epoxy were loaded but only 10 - 15 ml entered the tissue) the aortic valve closed immediately and no epoxy entered the ventricle). In the case of the dried porcine lung, some 350 ml of polyester resin were pumped in under pressure of 50 cm water and the interval of some 10 minutes, although here infiltration was poor due to the accumulation of numerous air bubbles in vessels of 5 - 7 mm diameter. Whether this is a characteristic of the polyester and/ or the lower infusion pressure has not been investigated, although none of the other specimens were troubled by air bubbles. A canine testicular artery was also attempted using 2.5m water pressure, but a rupture ensued at the level of the pampiniform plexus. It is suspected that poor washing (presence of blood clots) and excessive pressure contributed to this accident. This organ has not yet been repeated.
Fromm B, Graf J, von Hagens G: Demonstration of neovascularization of anterior cruciate ligament/ allografts utilizing the epoxy injection method. J Int Soc Plastination 5 (1): 3-6, 1991.
https://doi.org/10.56507/XWBQ9353
Pretorius WF, Geyer HJ: The use of E20 red resin for casting anatomical cavities. J Int Soc Plastination 9 (1): 37,1995. https://doi.org/10.56507/JQTR9706
Riepertinger A, Heuckendorf E: E20 color-injection and plastination of the brain. J Int Soc Plastination 7 (1): 8- 12, 1993. https://doi.org/10.56507/YHON8469
Stephenson RJ: Mechanics and Properties of Mattrer. 3rd Ed. New York, Willey. 375, 1969.