1Department of Anatomy, Cerrahpasa Medical Faculty, Istanbul University, Istanbul, Turkey
2Institute of Anatomy, Medical University of Graz, Graz, Austria
During an educational demonstration of the P35 technique, brain slices which had been immersed in P35 resin and stored in a cold room (5° Celsius) for approximately two years were used. The resin was very viscous and it was difficult to remove the steel basket containing the brain slices from the container of resin. There were technical difficulties during the manipulation of the slices: slices were brittle and fragile, filter paper spacers were stuck to the specimens, curing had begun where the slice touched the grid and gel-like resin remnants were stuck on the metal grids. Despite the very long immersion period and the problems encountered, the final specimen was satisfactory from an optical point of view.
plastination; P35; second immersion; impregnation
M. ÜZEL: Department of Anatomy, Cerrahpasa Medical Faculty, Istanbul University, Kocamustafapasa, Istanbul, 34098, Turkey, Tel: +90-532-2637425; Fax: +90-212-2470298; E-mail: muzel@istanbul.edu.tr
![]()



The well-known classic P35 method is the most used basic technique for brain slice plastination. It is used to obtain semitransparent brain slices, and yields excellent gray-white matter distinction. Its main steps include: fixation with formaldehyde, slicing and placing on stainless steel grids, flushing & precooling to +5°C, two dehydration baths of 2-4 days, two immersion baths (1 day each), forced impregnation, casting in double glass chambers, light curing, heat curing, and finishing (Weiglein, 1996; Weber et al., 2007). By completing all of these steps, the final product is a beautiful, durable brain slice helpful for studying sectional anatomy of the brain.
In this case report, the optical quality of the end product, as well as the technical experience after a very long duration (two years instead of one day) in the second immersion bath of the P35 method are shared.
Technical case report
During an educational demonstration of the P35 technique at the Institute of Anatomy, Medical University of Graz, Graz, Austria, the brain slices that were to be used were placed in the second immersion bath (P35/A9 mixture) approximately two years previously and were stored in the immersion bath during this period at +5°C. The normal methodology would be to commence impregnation after the slices have been in the second bath for 1 day. Therefore, it was logical to continue the plastination process with 24 hours of forced impregnation of the brain slices which were submerged in this two-year-old resin-hardener (P35-A9) mixture. After the vacuum/impregnation was completed, the slices were removed from the basket/polymer and positioned on the glass, and a double glass chamber was constructed. The chambers were filled with fresh resin mix (P35/A9), the slices were positioned with a wire, and the slices were then cured using UV-A light and heat.
Observations
Problems were observed from the beginning. After storage and vacuum application, the resin from the immersion bath was too viscous and it was very difficult to remove the steel basket of impregnated slices from the resin. After a struggle, the basket was removed. The slices were rigid, fragile, brittle and difficult to handle (unfortunately some of the slices broke into pieces) (Fig. 1). The filter papers between the slices were often united with the slices. With a lot of effort, most of the papers were removed from the slices. Portions of the slices had started to cure, and gel-like resin remnants were stuck to the metal grids (Fig. 2). Also, the grids left marks on some of the specimens (Fig. 3). Because of their fragility and the increased viscosity of the resin, it was difficult to put the slices onto the glass for construction of the double glass chambers without breaking them. After assembling the double glass chambers, the chambers were filled with fresh P35/A9 mixture and the slice position was adjusted with a wire. The hardening procedure was the usual UV-A light-heat combination. Some finished specimens had excavations and traces of filter paper on them. Despite the very long immersion period (two years) and the problems encountered, the final specimens were optically satisfactory (Fig. 4).
 Figure 1: P35 broken impregnated brain slices. |
 Figure 2: Remnants of (P35/A9) resin mix on the metal grids. |
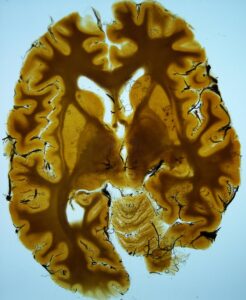 Figure 3: Cured P35 brain slice. |
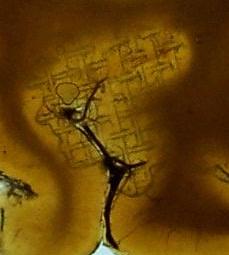 Figure 4: Grid marks on finished specimen. |
The duration of the second immersion step of the P35 method is normally one day. In this case, the specimens were forgotten and unintentionally remained in the second immersion bath (in the cold room) for two years. Because of this very long second immersion period, problems were encountered during the remaining P35 procedure. The problems (i.e. brittle, fragile, and partially cured specimens and gel-like resin sticking on the metal grids) encountered during the handling of the specimens were probably due to the very long (two years) exposure time of the specimens to acetone (von Hagens, 1986) and the increased resin viscosity. The acetone or length of time of the resin and catalyst being mixed likely caused a reaction to change the polymer into a gel.
In conclusion, the time for production of brain slices with the P35 method can be extended up to several months by storing the slices in a cold immersion bath. However, too long a duration should be avoided because the slices start to cure at the regions where they are directly in contact with the steel grid, and viscosity of the resin mix increases markedly. These findings suggest that in the P35 method, brain slices can stay in the second immersion bath for months without having any decrease in optical quality of the final specimen. On the other hand, changes in the physical properties of the resin should be expected which will result in mechanical problems that plastinators should be prepared to deal with.
von Hagens G, 1985: Heidelberg Plastination Folder, 2nd English Edition: Collection of all technical leaflets for plastination, pg. 3:9-10. Biodur Products, D-69126 Heidelberg, Germany
Weiglein AH, 1996: Preparing and using S-10 and P-35 brain slices. J Int Soc Plastination 10(1): 22-25.
https://doi.org/10.56507/IXGV4189
Weber W, A Weiglein, R Latorre, RW Henry, 2007: Polyester plastination of biological tissue: P35 technique, J Int Soc Plastination 22: 50-58.
https://doi.org/10.56507/MFED4472