1 Service d'ORL et de Chirurgie Cervico-Faciale, CHUV, 1011 Lausanne, Switzerland.
2 Service de Radiologic, CHUV, 1011 Lausanne, Switzerland.
3 Institut de Biologie Cellulaire et de Morphologie, Universite de Lausanne, Rue du Bugnon 9, 1005 Lausanne, Switzerland.
Ethmoidal regions were prepared and dissected to demonstrate regional sinus anatomy and endoscopic surgical approaches from six human heads. After preparation, the specimens were plastinated using the standard S10 technique. A CT-scan of each ethmoidal block was performed before and after preparation of the block to access shrinkage. The plastinated specimens were successfully introduced into clinical teaching of sinus anatomy and surgery. One advantage of using these specimens is their long-lasting preservation without deterioration of the tissue. The specimens are well suited for comparative radiographic and endoscopic studies, and the CT-scans allowed an exact measurement of tissue shrinkage due to plastination. Increased tissue rigidity and shrinkage due to plastination has to be taken into account for subsequent endoscopic observation.
anatomy; computed tomography; endoscopy; plastination; radiology; sinus; surgery
B.M. RIEDERER: Telephone: 41-21-692 5154; Fax: 41-21-692 5105; E-mail: BeatMichel.Riederer@ibcm.unil.ch
![]()



In otorhinolaryngology endoscopic sinus surgery bears a potential danger of severe hemorrhage in the nasal, orbital and intracranial regions (Stankiewicz et al., 1987; Stammberger et al., 1990). To be able to perform such surgery, it is necessary for the surgeon to train on ex vivo ethmoidal blocks (EB), which have been previously removed from cadavers and dissected. However, it is increasingly difficult to obtain human bodies. For this reason, development of a reusable didactic tool that favors the study and the understanding of anatomical structures of the sinuses and the nasal cavity, as well as the study of the principles of basic surgical interventions is imperative. Plastination or polymer impregnation of tissue should render the ethmoidal blocks long lasting, without tissue decay. Furthermore, the use of plastinated EB should decrease the number of EB that have to be prepared for training programs each year. This report outlines a method of preparing plastinated EB for teaching, while highlighting some aspects related to plastination. Shrinkage of the nasal mucosa and the bony ethmoidal labyrinth was measured to see if tissue changes due to plastination modify endoscopic observations. The value of plastinated EB was examined for teaching by associating the reading of a CT-scan of the EB to the direct observation of the EB.
Six cadavers were obtained from the local donation program at the Institute of Cell Biology and Morphology, University of Lausanne. Fresh human heads were used for the preparation of ethmoidal blocks according to the following protocol. The maxillary and internal carotid arteries were exposed, cannulated and injected following the procedure of Musumeci and colleagues (2003). Heads were frozen at -20°C and sliced on a band saw horizontally into three pieces (Fig. 1A). The saw lines were parallel to a line joining the external acoustic meatus to the external (lateral) canthus of the eye (dotted line). The middle block was saved for preparation of the EB and allowed to thaw to remove the brain. After removal of the brain, the block was frozen again. Further block preparation was by sawing vertically through the middle of the sella turcica (Fig.1B) and by removing the external (lateral) quarters of the face by sawing along a line passing through the middle of the orbit (Fig. 1C). From the six heads, two remained as complete blocks, while four were separated into eight hemiblocks by sawing on the midline and removing the nasal septum (Fig. 1C). After sawing of the ethmoidal blocks, they were stored in 50% ethanol at room temperature to await the intended surgical dissection. Six frequent endoscopic sinus surgeries were performed on the ethmoidal blocks: antrostomy, unciformectomy, frontomeatotomy, anterior ethmoidectomy, posterior ethmoidectomy with sphenoidotomy and complete ethmoidectomy. On eight hemiblocks the following combinations of surgerical interventions were performed: two antrostomies; one antrostomy and unciformectomy; two antrostomies, unciformectomies and frontomeatotomies; one antrostomy, unciformectomy, frontomeatotomy and anterior ethmoidectomy; one posterior ethmoidectomy and sphenoidotomy; and on one hemiblock and two complete blocks, all six procedures were performed. An anterior middle turbinectomy was done to gain access to the middle meatus. A dissecting microscope and an endoscope were used for surgical interventions.
Multirow detector helical computer tomography (MRCT) was performed with a Lightspeed QX/i Scanner (GE Medical Systems, Milwaukee, Wis, USA). The scanning parameters used for MRCT were: 120 kVp, 200 mAs, FOV - 16, section thickness - 1.25mm, and image data was reconstructed at 0.7mm intervals. The reformatted images were transferred to a GE Advantage Workstation (version 4.0), where 2D coronal and sagittal views were obtained at 0.5mm intervals and photographed with a wide window width (3,500 UH) centered at +400 UH to enable precise analysis of the bony structures. CT-scans were made after surgery (while specimens were held in 50% alcohol) and after plastination using similar parameters.
The following parameters were measured on magnified CT-scan images from each EB before and after plastination (Fig. 2): 1. Intersphenoidal width, 2. Nasal bone to vomer height, 3. Width of posterior nasal fossa, 4. Mucosal thickness of medial wall of concha bullosa, 5. Intersinusonasal wall to medial side of inferior concha, 6. Sagittal axis of maxillary sinus. These measurements are listed in Table 1.
|
Parameter measured |
Measurement before plastination |
Measurement after plastination |
Bone and mucosa shrinkage |
|
| 1 | Intersphenoidal width | 27.9 mm | 27.8 mm | 0.36% |
| 2 | Nasal bone to vomer | 49.3 mm | 49.2 mm | 0.2% |
| 3 | Width of posterior nasal fossa | 5.3 mm | 5.3 mm | 0% |
| 4 | Mucosa - medial wall of concha bullosa | 2.4 mm | 2.3 mm | 4.1% |
| 5 | Intersinusonasal wall to medial side of inferior concha | 10.0 mm | 10.0 mm | 0% |
| 6 | Sagittal axis of maxillary sinus | 43.8 mm | 43.7 mm | 0.23% |
Plastination of specimens was done using the standard S10 method (von Hagens, 1985; Henry and Nel, 1993; Weiglein and Henry, 1993). The steps taken and their associated time intervals were: dehydration was performed at room temperature using a graded series of technical alcohol of increasing concentrations from 50% to 100%, changed every third day with a final three changes in 100% ethanol. Ethanol dehydrated specimens were placed into 95% acetone at 4°C for 24 hours and then into 100% acetone at -20°C which was changed three times at three day intervals. Specimens were submerged in -20°C silicone polymer (10 Biodur®) containing 1% catalyst (S3 Biodur®) for 24 hours. The next day, vacuum was applied and impregnation was started by reducing air pressure gradually over a period of 4-5 days. After impregnation was complete, excess silicone was drained first at - 20°C, then at room temperature. After draining, excess silicone was wiped off with gauze and paper towels before and during the early phases of curing with S6 (Biodur®). During curing, openings into cavities were filled with gauze to prevent pooling of polymer and ensure the openings of the nasal cavities remained patent to allow entry of the endoscope later. After curing, landmarks, which are delicate and crucial, were marked on several specimens with colored nail polish. These marks were coated with a silicone-mix and hardened.
After curing, no morphological changes were observed and anatomical detail was still visible, even six months after plastination of the blocks. The plastinated concha were more rigid than the 50% alcohol fixed tissue. The shrinkage of the mucosa 6f the EB and the ethmoidal labyrinth was 0 to 5% (mean value: 2.3%) and 0 to 3% (mean value 1%) respectively during dehydration and plastination.
From the six heads, two were not separated and remained as complete blocks, while four were prepared into eight hemiblocks and the resultant specimens were of equal quality. Of the six surgical approaches utilized, the quality of the plastinated end products were similar. The vertical midline cut removed the anterior turbinate.
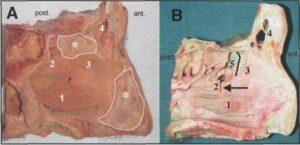
Figure 3. Left hemiblocks: A. Before surgery and plastination. B. After surgery (unciformectomy, anterior ethmoidectomy, frontomeatotomy) and plastination. 1. Inferior turbinate, 2. Middle turbinate, 3. Uncinate region, 4. Frontal sinus, 5. Hollow ethmoid bulla, * with white outline indicates remaining nasal septum.
The EB in figure 3 demonstrates before and after surgical intervention of an unciformectomy, anterior ethmoidectomy and a frontomeatotomy. The anatomical changes produced by the surgery are easily seen, since the bony parts are missing. The anterior middle turbinectomy allowed easy access to the middle meatus.
Tridimensional rhinosinusal anatomy is shown in figures 4 and 5. For each dissected block, pre- and postoperative CT-scans provided a three-dimensional correlation of the radiographic anatomy (Fig. 6). Vascular injection of the internal carotid, ophthalmic and ethmoidal arteries via the maxillary artery highlighted the vessels so that they were visible to the naked eye as well as radiographically. The marking of the less resistant bony areas of the ethmoidal labyrinth was also clearly visible.
This work demonstrated that plastination with the S10 standard method described by von Hagens (1985) is applicable for preparing human EB. However, we recommend using the described cutting lines (Fig. 1) to make a useful block. The resection of the head of the middle turbinate was done prior to plastination to gain access to the ethmoid, because the post mortem specimen doesn't offer the same tissue elasticity as the living patient. The absence of the anterior turbinate also helps visualize the underlying ethmoidal surgery. Unciformectomy, anterior ethmoidectomy and frontomeatotomy consist of hollowing out the uncibullar complex and enlarging the nasofrontal canal, absolutely respecting the lamina papyracea and the ethmoidal roof. It is evident that the blocks allow a better comprehension of the difficult tridimensional anatomy of the sinus and show in a glance what must be the result of such an operation.
Sinus plastination has been performed and published using the entire EB in situ of birds (Henry et al., 1997) and axial slices of human sinuses (Sprinzl et al., 1995) for purely descriptive purposes. A recent publication by Durand and coworkers (2001) provides an example of a plastinated model of maxillary sinuses that was used to test aerosols.
Although the standard S10 procedure described by von Hagens (1985) was used, there are several key points worth mentioning. Before curing, it was important to drain and wipe off any excess silicone in order to obtain empty sinus cavities. As well, to keep the nasal cavity, sinuses and openings free of polymer and open, it was important to fill spaces and openings with gauze and change as needed.
The plastination of EB presents numerous advantages. The blocks are odorless, dry, reusable, more robust and durable for years. Furthermore, because shrinkage of the nasal mucosa or of the ethmoid labyrinth could change nasosinus anatomy and endoscopic observation, pre and post plastination measurement of the bone and mucosa were recorded. We observed that the average bony and mucosal shrinkage was small (1% and 2.3%, respectively). In previous studies, tissue shrinkage in plastination was reported to vary between 8.2-29% (Brown et al., 2002). These values are quite different from those reported here, but Brown and colleagues reported results from kidney, liver and hearts from various species, while we focused on bony structures. This can easily explain a difference in shrinkage. Thus, plastination does not significantly shrink or distort the EB, and endoscopic appearance remains fairly realistic. Nevertheless, the loss of natural elasticity due to this technique makes endoscopic observation slightly more difficult.
For trainees in otorhinolaryngology, learning surgical techniques (especially those techniques associated with endoscopic sinus surgery) is critical to understand the regional anatomy because complications, although rare, can have disastrous consequences (May et al., 1994; Sharp et al., 2001). Surgical intervention is often required when conservative treatment of chronic infectious sinusitis, nasal polyposis, mucocoele or acute sinusitis fails. These six techniques (antrostomy, unciformectomy, frontomeatotomy, anterior ethmoidectomy, posterior ethmoidectomy with sphenoidotomy and complete ethmoidectomy) demonstrated on EBs are the most common procedures performed in endoscopic sinus surgery. Anatomical study of an EB with the naked eye or microscope, combined with endoscopic observation gives an excellent tridimensional view of the operative principles and their limits. Moreover, by marking less resistant bony areas of the ethmoidal labyrinth, areas for potential surgical complications are highlighted and areas that must always be preserved are pinpointed. Awareness of areas of potential operative complications was aided by injecting the internal carotid and maxillary arteries with a substance that rendered the regional vessels visible to the naked eye as well as radiographically (Musumeci et al., 2003) and by marking with colored nail polish the less resistant bony areas of the ethmoidal labyrinth (Waridel et al., 1997). These spots of color were coated with a thin film of silicone to increase their resistance to mechanical insult (Fig. 6). The optic canal, the wall of which has a resistance three times greater to the most fragile bony areas of the labyrinth, is not represented in figure 6. Nevertheless, breaking of the anterior wall of the optic canal with a resulting lesion of the optic nerve is one of the most serious complications of endoscopic sinus surgery (May et al., 1994). These marked areas alert the physician during endoscopic training to use caution when approaching these delicate areas of the plastinated nasal cavity.
The use of the endoscopic and CT-scan observations enhanced the study of tridimensional rhinosinusal anatomy and allowed the different steps of the surgery to be followed easily. The study of plastinated EB gives a more realistic understanding of ethmoidal anatomy and adds a tool that seems superior to books of clinical, radiographic and tridimensional rhinosinus anatomy, including its numerous variations and optic nerve and internal carotid artery precautions. Although the higher rigidity of plastinated tissue rendered endoscopic observations of EB slightly more difficult. EB in clinical teaching may be used in surgery, endoscopy and radiology. The training on ex vivo specimens (EB) remains fundamental. However, the use of plastinated EB should simplify and shorten the training phase and contribute to diminishing the number of cadaver heads needed for the apprenticeship of endoscopic sinus surgery and in the future decrease potential surgical complications.
Since sinus surgery is currently performed primarily using endoscopy, it is very important that the plastinated EB can also be examined endoscopically.
Acknowledgements: The authors thank Profs. P. Monnier and F.P. Harris for their constructive comments. This work was supported by the IBCM. The first author thanks his wife and children for their moral support.
Brown MA, Reed RB, Henry RW. 2002: Effects of dehydration mediums and temperature on total dehydration time and tissue shrinkage. J Int Soc Plastination 17:28-33.
https://doi.org/10.56507/XNQM4606
Durand M, Rusch P, Granjon D, Chantrel G, Prades JM, Dubois F, Esteve D, Pouget J-F, Martin C. 2001: Preliminary study of the deposition of Aerosol in the maxillary sinuses using a plastinated model. J AerosolMed 14:83-93.
https://doi.org/10.1089/08942680152007936
Henry RW, Antinoff N, Janick L, Orosz S. 1997: E12 Technique: an Aid to Study Sinuses of Psittacine Birds. Acta Anat 158:54-58.
https://doi.org/10.1159/000147911
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7(1):27-31.
https://doi.org/10.56507/WUXP9436
May M., Levine H. L., Mester S. J., Schaitkin B. 1994:Complications of Endoscopic Sinus Surgery: Analysis of 2108 Patients - Incidence and Prevention. Laryngoscope 104:1080-1083.
https://doi.org/10.1288/00005537-199409000-00006
Musumeci E, Lang FJW, Duvoisin B, Riederer BM. 2003: Plastinated ethmoidal region: II. The preparation and use of radio-opaque artery casts in clinical teaching J Int Soc Plastination 18:29-33.
https://doi.org/10.56507/XOZM8946
Sharp HR, Crutchfield L, Rowe-Jones JM, Mitchell 2001: Major Complications and Consent Prior to Endoscopic Sinus Surgery. Clin Otolaryngol 26:33-38
https://doi.org/10.1046/j.1365-2273.2001.00394.x
Sprinzl GM, Eckel HE, Sittel C, Thumfart WF, Koebke 1995: Ganzorganplastination in der Hals-Nasen- Ohrenheilkunde. HNO 43:282-286.
Stammberger H, Posawetz W. 1990: Functional Endoscopic Sinus Surgery. Eur Arch Otolaryngol 247:63-76.
https://doi.org/10.1007/BF00183169
Stankiewicz, JA. 1987: Complications of Endoscopic Intranasal Ethmoidectomy. Laryngoscope 97:1270- 1273.
https://doi.org/10.1288/00005537-198711000-00004
von Hagens G. 1985/1986: Heidelberg Plastination Folder. Anatomisches Institut I, Universitat Heidelberg, D-6900 Heidelberg.
Waridel F, Monnier P, Agrifoglio A. 1997: Evaluation of the Bone Resistance of the Sphenoid and Ethmoid Sinuses. Laryngoscope 107:1667-1670.
https://doi.org/10.1097/00005537-199712000-00017
Weiglein A, Henry RW. 1993: Curing (Hardening, Polymerization) of the polymer - Biodur S10. J Int Soc Plastination 7(l):32-35.
https://doi.org/10.56507/ABNZ7085