1- Service d'ORL etde Chirurgie Cervico-Faciale, CHUV, 1011 Lausanne, Switzerland.
2- Service de Radiologic, CHUV, 1011 Lausanne, Switzerland.
3- Institut de Biologie Cellulaire et de Morphologie, Universite de Lausanne, Rue du Bugnon 9, 1005
Lausanne, Switzerland.
In endoscopic sinus surgery, knowledge of the course of the internal ethmoidal and orbital arteries is crucial. The maxillary and the internal carotid arteries of cadavers were injected with radio-opaque, red colored silicone. The ethmoidal regions were prepared and plastinated using the standard S10 technique. On some specimens, the ophthalmic and ethmoidal arteries were dissected prior to plastination. The plastinated specimens of the ethmoidal blocks were successfully introduced into clinical teaching of sinus anatomy and surgery as an aid to study vascularization and its relationship to surgical procedures. Among the advantages of this method are the long- lasting preservation of dissected tissue, visualization of arteries during endoscopic and radiological examinations, and invaluable teaching and training resources for endoscopic sinus surgery.
anatomy; endoscopy; plastination; radiology; sinus; surgery
B.M. RIEDERER: Telephone: 41-21-692 5154, Fax: 41-21-692 5105; E-mail: BeatMichel.Riederer@ibcm.unil.ch
![]()



Knowledge of the exact location of the orbital and internal carotid arteries is crucial when performing endoscopic sinus surgery. In fact, injury of one of these arteries can provoke life-threatening hemorrhage from the nose or in the orbit or brain, which can lead to permanent damage despite emergency operative measures. In order to perform such surgery, it is necessary that surgeons train on cadaveric material. In this study, arteries of the ethmoidal region were injected with radio-opaque, colored silicone and ethmoidal blocks (EB) were prepared. After EB were prepared, the arteries were dissected to provide better visualization. Plastination was chosen to permanently preserve the injected EB. The plastinated EB help provide an understanding of various surgical procedures and potential complications that can occur when arteries are damaged. The advantages of plastinated EB for teaching are reported. Special reference is given to visualizing the ophthalmic and ethmoidal arteries on anatomical preparations using the naked eye, the endoscopic and radiographic techniques.
Cadavers with prior written consent were obtained from the local donation program at the Institute of Cell Biology and Morphology. For the preparation of casts, fresh heads, which are better than embalmed tissue, were used. A mixture of radio-opaque, colored silicone was prepared from 57% dense silicone (Elastosil® M 4500, Wacker-Chemie GmbH, 81737 München, Germany), 40% silicone oil (AK 50®, Wacker-Chemie GmbH, 81737 München, Germany) and 3% hardener (Wacker T12®, Drawin Vertriebs-GmbH, 85521 Ottobrunn, Germany). To this solution, 3% red pigment (SAL 300®) and 5% lipophil iodized solution (Lipiodol® ultra-fluid = Ethiodol® in the USA; Laboratoire Guerbet, 93600 Aulnay-Sous-Bois, France) were added. In order to determine homogeneity of this radio-opaque solution of silicone, mixtures of varying concentrations (50, 25, 10, 5, 1%) of Lipiodol were prepared and placed in 1.2mm id catheters and evaluated visually and radiographically.
After the best concentration of radio-opaque, colored silicone was determined, the heads were injected with 5% Lipiodol used to make the colored, radio-opaque silicone solution. The left and right maxillary and internal carotid arteries were exposed and catheterized with flexible plastic tubing (2mm id). These arteries were filled with 100 to 200ml of the silicone solution using 60ml syringes attached to the tubing. Because of vascular resistance and the viscosity of the solution, injection time was rather long (8 to 10 minutes). Hence, a syringe equipped with a common caulking gun was used to decrease injection time and assure vascular filling. Filling was stopped when the solution came out of the internal jugular vein and the anterior and posterior spinal veins. This caulking is available in do- it-yourself shops or hardware stores and makes injection easier and forceless.
After injection, the heads were stored in a refrigerator at 4°C for two days while the silicone hardened. After the silicone mix hardened, the heads were frozen at -20°C and cut with a band saw following the protocol described by Musumeci and coworkers (2003). The brain was removed and the block was frozen again. The ethmoidal blocks were cut and kept in 50% ethanol at room temperature during preparation of the surgical interventions and performing CT scans of the EB. A CT-scan was performed prior to surgical intervention with a Lightspeed QX/i Scanner (GE Medical Systems, Milwaukee, WS, USA). The scanning parameters used for multi-detector row CT were: 120 kVp, 200 mAs, FOV - 16, section thickness 1.25mm and image data were reconstructed at 0.7mm intervals. The reformatted images were transferred to a GE Advantage Workstation (version 4.0). At the workstation, 2D coronal and sagittal views were obtained at 0.5mm intervals and imaged with a wide window width (3,500 UH) and centered at +400 UH to enable precise analysis of the bony structures. To evaluate the opacified ethmoidal and ophthalmic arteries 3D images were reconstructed from the coronal and sagittal views using the volume rendering reformatting technique, which incorporates all relevant data into the resulting image. The vessels were colored red using Adobe Photoshop. A dissecting microscope and an endoscope were used for performing the various surgical interventions, such as unciformectomy, frontomeatotomy, ethmoidectomy or antrostomy and dissection of the orbital arteries. CT-scans were also performed after surgery and after plastination using the same parameters as described before. After the surgeries were completed, the specimens were plastinated using the classic cold S10 technique (von Hagens, 1985; Henry and Nel, 1993; Weiglein and Henry, 1993). CT scans were taken once again after plastination using the same parameters as above.
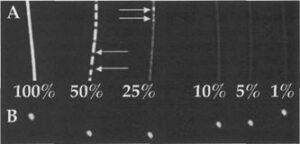
Figure 1. Longitudinal (A) and transverse (B) CT-scan of 1.2mm diameter plastic tubes filled with decreasing % concentrations of Lipiodol® in a colored silicone solution. Arrows - Uneven distribution of silicone and Lipiodol.
All percentages of the radio-opaque silicone solutions made from 5% Lipiodol as well as pure Lipiodol were visible in the 1.2mm diameter tubes with the eye and on CT scan (Fig. 1). The concentrations of 50% and 25% of Lipiodol® did not remain homogenous. The 1 % solution showed less radio-opacity and was less visible than the 5 and 10% solutions.
Injection of the radio-opaque colored silicone resulted in good opacification of the orbital and internal carotid arteries in all four cadaver heads (Figs. 2, 3). As well, the injected arterioles, veins, and capillaries of the skin and mucosa of the hemiblocks were red (Figs. 2, 3, 4). The difference in the tissue color between an injected and non-injected ethmoidal block (EB) is demonstrated in Figure 2.
After injection and prior to dissection, the trajectories of the ophthalmic, anterior and posterior ethmoidal and internal carotid arteries were visible on the three dimensional CT-scan reconstructions (Fig. 4). After dissection of the orbital arteries and after anterior and posterior ethmoidectomy, all arteries could be seen with the naked eye, radiographically and endoscopically (Figs. 4, 5). The arteries of the orbit, the stump of the optic nerve, the ophthalmic and the supratrochlear arteries are recognizable, as well as the anterior ethmoidal artery with its entry in the lamina papyracea. In one ethmoidal block, aggregation of color was noted after plastination (Fig. 6). The color enhancement of the reconstructed 3D images from the CT scans rendered the vessels quite prominent such that the anterior ethmoidal artery is seen on the roof of the ethmoid (Fig. 4B). Transillumination of ethmoidal roof on EB (Fig. 5B) aided identification of the thin ethmoidal arteries in their bony canals.
Vascularization of the ethmoidal sinus region is of clinical relevance and must be known before practicing endoscopic sinus surgery. Hemorrhages, such as orbital hematomas (pre- and postseptal), epistaxis after sinus surgery and focal brain hemorrhage, represent a large percentage of minor and major complications (Stankiewicz et al., 1987; May et al., 1994). Epistaxis can be cataclysmic after injury of the internal carotid artery. In a recent study, Sharp and colleagues (2001) reported that the incidence of major and minor hemorrhagic complications represented 0.04% and 0.44% of all endoscopic sinus interventions, respectively. Knowledge of the trajectories of the ethmoid, sphenopalatine and internal carotid arteries is therefore primordial for successful prevention of potential hemorrhagic complications.
An iodinated colored silicone solution was needed to highlight the vessels of the region so that the vessels could be seen by eye as well as radiographically. It was necessary to determine the percent of iodine needed. Of the 5 mixtures with silicone compounded and the one pure solution, 5% Lipiodol® solution was chosen to highlight the vessels as it was visible in the thin (1.2mm) tubing, remained a homogeneous solution, and did not interfere with the hardener of the silicone. Concentrations of 50% and 25% of Lipiodol® were not stable and did not remain homogeneous. However, with macroscopic, endoscopic or radiographic observations the vessels were still distinguishable.
After injecting cadaver heads with the colored, radio-opaque solution, dissecting the orbit, and splitting the ethmoidal block in two hemiblocks, the arterial vascularization of the medial wall of the orbit and of the ethmoid are clearly demonstrated. Although the ethmoidal arteries have a diameter of less than lmm, they were always recognizable by visual or endoscopic inspection. If necessary, transillumination of the EB can enhance the contrast of the injected arteries and improve observation and learning of surgical anatomy. After plastination, uneven coloration of arteries was observed in one ethmoidal block, probably due to accumulation and aggregation of injected material during the plastination process.
The use of arterial casts with a radio-opaque solution is helpful in both the understanding of arterial vascularization and allowing surgeons to be trained more easily. It facilitates the study of the relationships of the arteries to other anatomical structures and allows a comparison with the CT scans which are usually performed prior to endoscopic sinus surgery. The reddish color of the facial skin and nasal mucosa illustrates the excellent quality of the injection which also enhances the aesthetics of the EB.
Study of ethmoidal blocks should be recommended in a surgical training program before execution of the first surgical exercises on a cadaveric ethmoidal block. For this reason, plastination is especially useful because it makes such an anatomic preparation permanent and reusable for multiple training courses even though preparation of an injected and plastinated block is time consuming. Indeed, the preparation of the radio-opaque solution and the anatomical dissection are easy, as well as injection of the maxillary and internal carotid arteries, especially if a compression pistol is used. Even if the injected colored, radio-opaque solution settles after plastination, the arteries are still visible by the naked eye or on the CT-scan. The disadvantages of plastination is that the plastinated blocks are no longer re-operable once the various interventions such as unciformectomy, frontomeatotomy, ethmoidectomy or antrostomy have been performed, and they can be used only for demonstration purposes. Endoscopic observation of a plastinated complete block (but not hemiblock) is a little more difficult than in vivo observation, because the specimens are more rigid after plastination. However, the results of the most common surgical interventions are clearly demonstrable on both the plastinated EB and on CT-scans and serve as an excellent teaching and training tool.
Acknowledgments: The authors would like to thank Profs. P. Monnier and F.P. Harris for their critical comments. The authors thank for the institutional support of the IBCM. The first author thanks his wife and children for their moral support.
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7(1):27-31.
https://doi.org/10.56507/WUXP9436
May M, Levine HL, Mester SJ, Schaitkin B. 1994: Complications of Endoscopic Sinus Surgery: Analysis of 2108 Patients - Incidence and Prevention. Laryngoscope 104:1080-1083.
https://doi.org/10.1288/00005537-199409000-00006
Musumeci E, Lang FJW, Duvoisin B, Riederer BM. 2003: Plastinated ethmoidal region: I. Applications in clinical teaching and effects of tissue shrinkage. J Int Soc Plastination 18:23-28.
https://doi.org/10.56507/GILN1147
Sharp HR, Crutchfield L, Rowe-Jones JM, Mitchell DB. 2001: Major Complications and Consent Prior to Endoscopic Sinus Surgery. Clin Otolaryngol 26:33-38
https://doi.org/10.1046/j.1365-2273.2001.00394.x
Stankiewicz JA. 1987: Complications of Endoscopic Intranasal Ethmoidectomy. Laryngoscope 97:1270- 1273.
https://doi.org/10.1288/00005537-198711000-00004
von Hagens G. 1985/1986: Heidelberg Plastination Folder. Anatomisches Institut I, Universitat Heidelberg, D-6900 Heidelberg.
Weiglein A, Henry 1993: Curing (Hardening, Polymerization) of the polymer - Biodur S10. J Int Soc Plastination 7(l):32-35.
https://doi.org/10.56507/ABNZ7085