1 Department of Anatomy, Dalian Medical University. No.9 west section, Lushun South Road, Dalian 116044, China
2 Dalian Hoffen Bio-technique Co., Ltd. No.36, Guangyuan Street, Lushunkou Economic Development Zone, Dalian 116052, China
Objective: To explore the procedure of preparation of a whole plastinated equine specimen to be used in veterinary education. Methods: A formalin-preserved horse was dissected to display the brain, spinal cord and the superficial muscles complete with their innervation. The specimen then underwent silicone impregnation. Results: The flexibility of the nerves and muscle tissues after plastination was maintained, and muscles as well as nerve structures were easily discriminated. The horse was positioned in a stance of a lively spring which facilitated exhibition of both dorsal and ventral structures. Conclusion: The silicone plastination technique produced a dry, odorless and durable specimen that is suitable for handling that will serve as an ideal whole equine specimen for veterinary anatomical education.
equine; nervous system; plastination; silicone impregnation; veterinary anatomy
Prof. Hong-Jin Sui, Department of Anatomy, Dalian Medical University, Dalian 116044, P.R. China. Fax.: +86 411 86110324; Email: sui@hoffen.com.cn
![]()



Plastination is the most important technique to have emerged in recent years for the preservation of biological specimens. Since its introduction in 1979, it has gained wide acceptance throughout the world (von Hagens, 1979). The most widespread application of this technique is in the preparation of a wide range of anatomical specimens for teaching, and it has been considered an important tool in recent proposals for the teaching of anatomy (Reidenberg et al., 2002, Latorre et al., 2007b, Valdecasas et al., 2009). Careful dissection of an embalmed animal can be a challenging, time-consuming process, and maintaining a dissected animal for a long period of time without deterioration due to fungal and microbial growth or desiccation is extremely difficult. Moreover, large animals that are immersed in fixative solution are not ideal for teaching and learning anatomy. The procedure described here employs silicone impregnation to preserve the original delicate tissues, and to furnish a dry, odorless and durable specimen of a whole large animal, in this case a horse.
The experiment using the horse was approved by Dalian Agriculture Bureau on Ethics in the Care & Use of Laboratory Animals. A freshly dead horse was collected from farmland. The right common carotid artery and jugular vein of the cadaver were cannulated and blood was washed out by introducing saline by gravity feed from a height of approximately 2.5m. This was followed by 10% formalin to fix the whole body. The animal was then stored in a tank containing the same solution for a period of over 6 months until dissection began.
Dissection
The superficial structures of the horse, including the skin, subcutaneous fat, fascia and blood vessels were removed to expose the muscles and their innervating nerves. In the head, the soft tissues covering the calvaria were removed, except for the ears. Craniotomy was performed to expose the brain. The branches of the facial nerve were carefully dissected out as they emerged from the rostral margin of the parotid gland. On the left side of the skull, bone was removed in order to expose the trigeminal ganglion and other cranial nerves. Dorsally, the epaxial muscles were removed to expose the vertebral arches, which were removed using a hand saw and chisel to open the vertebral canal and expose the spinal cord. After the brain and spinal cord had been uncovered, some cranial and spinal nerves were carefully dissected from adjacent structures. The contents of the thoracic and abdominal cavities were removed to facilitate the plastination process and to aid in preserving the stance of the horse. The total dissection time was about 800 hours. The viscera were plastinated separately before being returned to their proper location (see below).
Dehydration
The dissected horse was dehydrated by the freeze substitution method (von Hagens, 1986). The horse specimen was precooled at +5°Cin a cool room in order to avoid the formation of ice crystals when placed in cold acetone. It was then placed in the first bath of 85% acetone at -25°C for about one month, and then transferred into a second bath of 90% acetone at -15°Cfor about one month. The specimen was then submerged in 95% acetone at room temperature for about one month. Finally, it was submerged in 99.9% acetone at room temperature while the purity of the acetone was monitored daily, using a standard acetonometer. When the purity of acetone as determined by the acetonometer remained the same for three consecutive measurements, the specimen was moved to a fresh bath of 99.9% acetone until dehydration was completed.
Forced impregnation
The central and most important step in plastination is replacement of the intermediary solvent by curable polymers. This is achieved by means of forced impregnation under a vacuum. Briefly, the impregnation process was as follows. The dissected horse was removed from the acetone bath and transferred to a tank containing a silicone base material (Hoffen R1) plus 3% thickener (Hoffen R3) (Dalian Hoffen Bio-technique Co., Ltd.) at -15°C in a specially-designed large-scale deep freezer (Bai et al., 2010). The release of acetone gas bubbles was monitored while the absolute pressure was slowly decreased from atmospheric pressure down to 20 mm Hg, then 10 mm Hg, 5 mm Hg, and finally near to 0 mm Hg. Impregnation was considered complete when bubbling ceased. This process took approximately 2 months to complete. In order to accommodate the large size of the horse specimen, a vacuum tank and cantilever hoist were specially designed and constructed for this procedure. The dimensions of the vacuum bath were 3.0 x 2.0 x 1.3 m, giving a capacity of 7800 liters (Figure1).
Positioning and replacing the viscera
The horse specimen was removed from the impregnation tank and the excess silicone was drained and wiped off. The horse was then suspended in a sling while its limbs were placed in the desired position – intended to recreate a dynamic posture of landing on its fore-legs. When the desired position was achieved, stainless steel rods were inserted through the joints to maintain the posture. Finally, the thoracic and abdominal viscera were returned to their previous positions within the thoracic and abdominal cavities, respectively (Figure 2).
Gas curing (hardening)
After positioning and replacing the viscera, the specimen was placed in a closed chamber at 35°C, and exposed to hardener vapor (Hoffen R6) (Dalian Hoffen Bio-technique Co., Ltd.) (Bai et al., 2010). A small peristaltic pump was used to bubble air through the hardener to form vapor and thus accelerate the curing. After one month, the specimen was completely cured. The muscles were colored using a proprietary brand of water-based paint and a soft-tissue brush. The coloring can be repeated after about six months if necessary when the color fades.
The result was a clean, dry, odorless, and durable real whole horse with a stance of a lively spring on the forelegs (Figure3). The superficial muscles, brain, spinal cord and some nerves were clearly displayed in situ. The positioning of the horse, with the rear legs raised, facilitated the display of both dorsal and ventral structures.
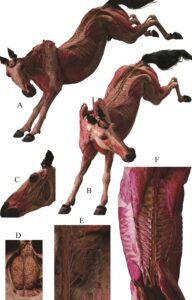
Figure 3 - The finished specimen is shown with a dynamic posture of landing on its forelegs. It was clean, dry, odorless, durable - and real. It clearly displays the muscular and nervous systems. A, lateral view of the horse; B, anterolateral view ; C, facial nerve; D, brain; E, sacral plexus; F, spinal cord and spinal nerves.
The technique of plastination consists of slowly replacing tissue fluids and a portion of the tissue lipids with a curable polymer, under vacuum. In this study, we prepared a whole horse plastinated specimen by the silicone impregnation technique, for veterinary anatomy education. The result is a clean, dry, odorless, lifelike and durable real biological specimen that can be handled without gloves and which does not require any special storage conditions or care. Plastinated specimens also have the advantage of sparing staff and students from exposure to the toxic substances used in the classical methods of embalming and preservation of biological tissues (e.g. formaldehyde, phenol and alcohols).
Up to now, plastination as a technique for preparing a variety of animal and human specimens has been extensively applied to education and research (Reidenberg et al., 2002; Latorre et al., 2007b; Valdecasas et al., 2009). In veterinary medicine, however, application of this technique has only just begun to develop (Latorre et al., 2007a, Latorre et al., 2007b). The importance of correlating anatomical studies with diagnostic and therapeutic approaches in practice has long been recognized. Such studies in the horse have, until recently, lagged behind this discipline in human medicine and surgery (Latorre et al., 2007a). Additionally, in the case of large animals such as the horse, only plastinated regional specimens or individual organs, such as the cephalic block, heart and transverse sections, have been available for use in evaluation of their effectiveness in anatomical education (Latorre et al., 2007b). A lack of whole large animal specimens may be detrimental to the students’ comprehension of whole-body animal anatomy. To address this lack, we endeavored to plastinate a whole dissected horse by the silicone-impregnation technique, in order to stimulate further correlations of anatomical structures and equine medical and surgical procedures, and thereby to advance knowledge and understanding in practice and teaching of equine anatomy.
Additionally, this work demonstrates that plastination with the silicone technique is applicable to the preparation of a whole horse specimen. In this study, Hoffen silicone R1/R3 was used for forced impregnation. The result was a clean, dry, odorless and durable real whole horse.
Acknowledgements
The authors would like to thank the technicians in Dalian Hoffen Bio-technique Co., Ltd. for their skillful technical assistance in silicone impregnation.
ai J,Gao HB,Jie L,Luan BY,Meng WJ,Zhang JF Yu SB,Gong J,Zhang CH,Sui HJ. 2010: The application of Hoffen P45 plastination technique on preparation of sectional specimen. Chinese J Clin Anat 28(1):107-108.
Latorre R, Rodríguez MJ. 2007a: In search of clinical truths: equine and comparative studies of anatomy. Equine Vet J 39(3):263-268.
https://doi.org/10.2746/042516407X192559
Latorre RM, García-Sanz MP, Moreno M, Hernández F, Gil F, López O, Ayala MD, Ramírez G, Vázquez JM, Arencibia A, Henry RW. 2007b: How useful is plastination in learning anatomy? J Vet Med Educ 34(2):172-176.
https://doi.org/10.3138/jvme.34.2.172
Reidenberg JS, Laitman JT. 2002: The new face of gross anatomy. Anat Rec 269(2):81-88.
https://doi.org/10.1002/ar.10076
Valdecasas AG, Correas AM, Guerrero CR, Juez J. 2009: Understanding complex systems: lessons from Auzoux's and von Hagens's anatomical models. J Biosci 34(6):835-843.
https://doi.org/10.1007/s12038-009-0097-0
Von Hagens G. 1979: Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 194(2):247-255.
https://doi.org/10.1002/ar.1091940206
Von Hagens G. 1985: Heidelberg Plastination Folder: Collection of technical leaflets for plastination. Heidelberg: Anatomiches Institut 1, Universität Heidelberg, p 16-33