1Department of Natural History, Royal Ontario Museum, Toronto, Ontario, Canada, M3S 2C6.
2Department of Anatomy and Comparative Pathology, University of Murcia, 30100 Murcia, Spain.
3Animal Health Center, Ministry of Agriculture, Abbotsford, British Columbia, V3G 2M3
4Marine Mammal Rescue, Fisheries Management, Fisheries and Oceans Canada, Vancouver, BC, V6C 3S4
There are few well-preserved heart specimens representing different whale species. The death in 2016 of an adult male southern resident killer whale (Orcinus orca) afforded an opportunity to plastinate an intact killer whale heart, using the standard von Hagens/S10-S3, cold impregnation technique. The process and technical challenges of plastinating such a large, hollow organ are presented. The result was a specimen of exceptional anatomical detail, amenable to highly resolved endoscopic and computerized tomography evaluation. Exhibited in a comparative context, the plastinated heart assures continued value as an object for education and a specimen of scientific interest.
Orcinus orca; plastination; heart; endoscopy; computerized tomography
Jacqueline Miller, MSc., Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Canada. Tel.: 414-586-5769; E-mail: jmiller@rom.on.ca https://www.rom.on.ca/en/collections-research/collections-natural-history
![]()



The importance of high quality and durable anatomical specimens has long been acknowledged by academic institutions, including schools of human and veterinary medicine. However, both quality and durability are of no lesser value to museum communities, where anatomical specimens are often integral to communicating certain content for natural history exhibitions. In such exhibits, when anatomical material is utilized as a teaching tool, there is a need for clear interpretation, as well as for engaging specimens. Typically, soft-tissue specimens are exhibited in a preservation fluid, which renders them minimally accessible to the museum visitor. Alternatively, a replica of a specimen may be sculpted using wax, clay, or synthetic materials with similar qualities, and then a model fabricated by molding and casting, or by employing 3D scanning techniques. While cost-effective and often physically detailed, such models are nonetheless illustrative, and may allow for teaching or research opportunities outside of the exhibition. Plastinates may serve as an alternative.
Plastination preservation replaces cellular and intercellular fluid with a curable polymer. The process results in touchable, non-toxic specimens of great longevity, rendering plastination as the gold standard for the preservation of rare or unique specimens (von Hagens 1986; Bickley et al., 1987; von Hagens et al., 1987).
Iconic, yet enigmatic, whales have experienced a rich evolutionary history of diversification spanning over 50 million years, resulting in remarkable physiological and anatomical adaptations to marine living (Gatesy et al., 2013). Among the physiological systems and associated organs bearing evidence of such adaptation, none is more interesting than the cardiovascular system, and especially the heart.
The cardiovascular system of small whale, porpoise, and dolphin species has been well characterized (Ivančić et al., 2014; Cozzi et al., 2017; Huggenberger et al., 2019) and there are also several detailed accounts of the heart for larger cetacean species (Race et al., 1959; Truex et al., 1961; Ochrymowych and Lambertsen, 1984; Tarpley et al., 1997). However, to our knowledge, the heart of the killer whale (Orcinus orca) has not been described, and a well-preserved killer whale heart would be of great value for anatomic and physiologic studies.
The killer whale is the apex marine predator of the Earth’s oceans and is iconic for its striking appearance, complex social behavior, and a cosmopolitan distribution (Ford, 2018). Overall, five distinct populations are recognized in North American coastal waters, and along the Northeastern seaboard of the United States and Canada, three ecotypes of killer whales currently occur (Bigg et al., 1990).

Figure 1. Southern resident killer whale (image courtesy Department of Fisheries and Oceans, Canada)
The resident killer whale ecotype includes a southern and northern population, with the southern resident killer whale (SRKW) (Fig. 1) designated as endangered under the Committee on the Status of Endangered Wildlife in Canada (COSEWIC, 2001 and 2008). In United States waters, all killer whales are protected under the Marine Mammal Protection Act (1972), and in 2005, the SRKW population was listed as endangered under the American Endangered Species Act (ESA) (National Marine Fisheries Service, 2008). A specialist consumer of Chinook salmon, the current number of SRKW is small and declining, with numbers estimated at a mere 74 individuals as of September 2020.
On March 30, 2016, a dead floating adult male killer whale, designated as L95, was located during a Department of Fisheries and Oceans (DFO) Cetacean Research Program Survey at 49° 32.5’N, 127° 13.8’W, west of Nootka Island off the west coast of Vancouver Island, British Columbia, Canada. The animal was secured by a tail line and towed ashore at Tahsis, BC, for necropsy on April 1, 2016. The whale presented in fair to moderate body condition with advanced post-mortem decomposition (code 3.5) and generalized sloughing of the skin. Despite the post-mortem state, the Royal Ontario Museum (ROM) wanted to obtain the intact heart to compare with the plastinated blue whale heart displayed in the ROM’s 2017 exhibition ‘Out of the Depths: The Blue Whale Story’. The blue whale heart was obtained after an unparalleled 2014 winter mortality event in the Gulf of Saint Lawrence, Canada. The animal presented with advanced post-mortem decomposition, with crush injury and freeze artifact, yet the heart was successfully plastinated using the standard S10/S3 cold impregnation protocol (Henry and Nel, 1993; DeJong and Henry, 2007; Miller et al., 2017; Henry et al., 2019). Based on the prior success with the blue whale specimen, the killer whale heart was collected, preserved, and prepared for plastination.
Specimen Removal
The killer whale (SRKW), L95, was necropsied on April 1, 2016, at Tahsis, British Columbia, Canada. The animal presented with advanced autolysis, with generalized sloughing of the epidermis and exposed blubber throughout the torso. The necropsy followed a standardized killer whale necropsy protocol (Raverty et al., 2014). Pathology results indicated that death was due to a generalized fungal infection (mucormycosis), secondary to a satellite tag deployment and detachment event where titanium tag pedicles remained in the animal at the base of the dorsal fin and formed a nidus of infection.
After external examination, the blubber and skin along the flank were removed. The abdominal musculature was incised along the costal arch and dorsal contour of the abdominal cavity, reflected, and the internal organs examined. The ribs were disarticulated at the costochondral junction and costovertebral articulations. The entire length of the intercostal musculature was cut, and each rib removed individually to expose the thoracic contents. The lungs and heart were assessed in situ, after which the pericardium was opened, and the great vessels truncated. The heart was exteriorized, placed in plastic bags, and frozen for shipment to the ROM. The frozen heart arrived the next day in a standard shipping cooler with ice, and was placed immediately into a -80˚ C freezer.
Specimen Preparation
Preparation began September 26, 2016. The frozen heart weighed 24 kg, and was thawed overnight, in a constantly flowing, 4-6o C tap water. Rinsing with cold water continued the next day, and the heart was gently massaged to remove blood clots and residue from the heart chambers and coronary vessels. The heart was fixed following conventional methods of immersion and dilation, and to some degree, perfusion (Oostrom, 1987a) beginning with 5% formalin. Fixative concentration was initially higher than the stepwise concentrations of 1 to 5% formalin recommended by Oostrom (1987a), in order to quickly arrest further decomposition of the heart once thawed. Final concentrations conformed to published recommendations (Henry, 1987). Dilation of the heart was achieved via vascular cannulation to each side of the heart (Tiedemann and von Hagens, 1982; Henry, 1987; Oostrom, 1987b).
A large, full thickness and irregular transverse defect in the region of the coronary sulcus required closure by suture during preparation, in order to successfully dilate the heart. Large openings in the dorsal wall of the left and right atria, corresponding to the outflow of the pulmonary veins from the lungs and compromised ostia of the caudal and cranial venae cavae (CdVC, CrCV), were occluded with plastic containers, and banded (Fig. 2).
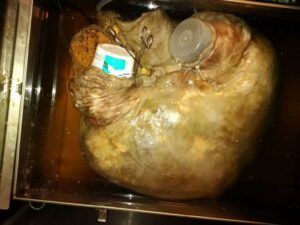
Figure 2. The dilated heart in formalin bath, with PVC cannulas clamped (via hemostats). Atrial basal aspect
The area affected by these defects, coupled with our desire to dilate the heart, made it necessary to suture another incision in the wall of the left atrium, at the origin of the pulmonary veins from the caudal lung lobes. A 15.9 mm (5/8 inch) OD PVC tube was inserted into the left atrium prior to banding. The root of the pulmonary trunk and the base of the aorta were preserved, then occluded with corks, and the pulmonary trunk banded. The elasticity of the aorta held the cork in situ without requiring ligature. A similarly-sized cannula was inserted into the coronary sinus dorsally and secured with a ligature. The heart was placed in a stainless-steel specimen storage tank (1 m x 1 m x 60 cm) for thawing, flushing, dilation, and fixation of the specimen.
The cannulated heart was immersed in an approximately 10 °C water bath, and the PVC cannulas were connected via latex rubber tubing to a submersible pump (Little Giant, Model 501004 #1, 115V-60Hz). Water was directed into the right and left heart chambers, alternately, under positive pressure (250 GPH) for continued flushing. The tap water was then exchanged with a formalin bath, in sequentially increasing concentrations of 5% (5 pbv stock solution formaldehyde 37% in 95 pbv tap water) for 5 days, then 8% (8pbv stock solution formaldehyde 37% in 92 pbv tap water) for 5 days, then a final concentration of 10-12% formalin (10-12 pbv stock solution formaldehyde 37% in 88-90 pbv tap water). The heart remained in this fixative concentration while in Canada. Alternate sides of the heart were dilated with formalin solution via either cannula. Cannulas were clamped when the chambers were fully dilated at each stage of fixation, and the heart was floated, buoyant in its bath, and covered in linen soaked with fixative. The heart was checked almost daily and, when necessary, was re-dilated and rotated in the fixative solution.
The circumference at the base of the heart (coronary sulcus) was approximately 131 cm when dilated, as measured roughly from the base of the R auricle to the area where the pulmonary arteries exit on the left side of the heart. As this large specimen precluded plastination at a Canadian facility, collaborators at the University of Murcia were approached and arrangements made to ship the preserved heart to Spain for plastination.
Packing and Shipping
In preparation for shipping, the formalin solution was drained, and the heart was thoroughly rinsed of fixative in tap water on October 19th, 2016. The heart was packaged and shipped in compliance with IATA guidelines as they apply to wet specimens of animal origin, similar to Miller et al. (2017). The heart was wrapped in wet absorbent paper towels and secured in a 2 mm plastic sheet. A second layer of plastic sheeting reinforced the initial wrap and formed the first receptacle. The bundle was then wrapped in a third layer of 2 mm plastic sheeting, with a layer of dry cotton roll interspersed between this and a fourth layer of 4 mm plastic sheeting. In accordance with the IATA guidelines, this formed the second shipping receptacle. An additional double layer of plastic ensured that the heart was completely sealed, and the bundle was placed in a large plastic cooler padded by bale cotton and foam peanuts. The cooler lid was sealed with silicone, and was the third, outer shipping receptacle. En route shipping temperatures were maintained between 10 and 20 °C.
Shipping specimens derived from or representing listed endangered species requires several permits. A Canadian letter of possession, a permit under Canadian Species at Risk legislation (SARA), as well as transport permits under the Convention on International Trade in Endangered Species of Flora and Fauna (CITES) for both ports of exit and entry (CITES permits 16CA02387/CWHQ, and ES-LA-00010/161) were required, and concerns regarding potential contamination or zoonoses were addressed by adherence to recommendations by Rutala et al. (2008).
The specimen arrived in Murcia on October 25th, 2016. The container was sealed, protected, and the contents were intact, showing
no signs of having been inspected. The heart was maintained moist through transit by the surrounding layers. On arrival, the atria were recannulated to allow circulation of fluid through the chambers of the heart. the aorta was accessed, the coronary arteries were cannulated (left and right, via the aortic trunk) and the heart submerged in running tap water (Fig.3) to allow the heart to recover its dilated shape. The heart was then transferred to fixation solution (10% formalin), re-dilated, and left for three weeks to attain the maximum degree of distension of the atria and ventricles.
After four weeks in formalin, the heart was transferred to a running water bath for 24 h, and subsequently the coronary arteries were injected via the sinus of the aorta with RVT silicone (Dow Corning), mixed with the 10% hardener and with BIODUR® AC52 red paste. To control the degree of distension of the coronary arteries, the injection was performed with manual pressure. The specimen was then refrigerated at 3 °C for 48 h before starting dehydration.
Dehydration
On November 22nd, 2016, dehydration in 96% acetone began at -25˚C (Fig 4). Acetone was circulated through the heart using a
peristaltic pump at the beginning of each acetone bath. After seven days, acetone purity was 90% and the heart was placed in a new acetone bath of 100% acetone. On December 9th, acetone purity was 96.5%, and the heart was subsequently placed in a third bath of 100% acetone. The next purity check (December 22nd) was 98.5%, therefore the heart was transferred to a fourth bath of 100% acetone. It was held at room temperature for 20 days to remove fat. Acetone purity was 99.3% in the last check prior to the start of impregnation. The acetone and the heart were then cooled to -18 °C to equal the temperature of the impregnation solution.
Impregnation (S10/S3)
Impregnation began on January 11th, 2017. The heart was immersed in the impregnation solution (BIODUR® S10B Reddish + S3 1%) at -18 °C and left at atmospheric pressure for 24 h (Fig 5). Pressure was then reduced progressively, as assessed by the rate of bubble formation on the surface of the impregnation solution, until a pressure of 20 mmHg was attained on day seven. At 14 days, the pressure was 10 mmHg, and after 19 days of impregnation, 5 mmHg was reached. Pressure was decreased and stabilized at 2 mmHg over five days. By day 25, surface bubbles were rarely observed indicating complete impregnation (Fig. 6). The heart was then repositioned in the chamber. The pressure of 2 mmHg was recovered and maintained for 3 more days. The specimen was then removed from the impregnation bath and left to drain the surface silicone for 2 days at room temperature.
Pre-curing & Positioning
While draining, the shape and distension of the heart were maintained by inserting paper towels into the lumina of the atria and ventricles. During this time, the main path of the coronary arteries was dissected. Removal of the serous pericardium (epicardium) along the coronary sulcus to near the subsinuosal interventricular sulcus exposed the right coronary artery and its ventricular branches (Figs. 7, 8). In the same way, the serous pericardial covering of the paraconal interventricular sulcus was removed to reveal the left coronary artery, including its paraconal inter-ventricular branch (Fig. 9). The great vessels at the base of the heart were identified and manicured to maintain correct topography, including the pulmonary trunk and the ascending aorta. The overall drainage of the pulmonary veins and venae cavae were likewise maintained, although their individual atrial ostia were compromised during the necropsy and extraction of the heart (Fig. 10).
Polymerization or Curing (S6 vapor exposure)
After a draining and positioning period of one week, polymerization, or curing, was completed with crosslinker S6 agent inside a gas container. After 2 days of S6 vapor exposure the surface of the heart was dry enough to create a window in the free wall of the right ventricle to reveal the internal morphology (Fig. 8). After 2 weeks of curing the heart was suitable for shipping back to Canada.
Completion Time
| Step of the protocol | weeks |
| Preparation of the specimen | 4 |
| Dehydration | 4 |
| Defatting | 3 |
| Impregnation | 4 |
| Positioning | 1 |
| Curing | 2 |
Imaging
The quality of the preserved specimen, and its significance as a rare taxonomic example, furnished an opportunity for additional study. Computerized tomography (CT) and endoscopic examination of the plastinated heart were therefore performed in the Minimally Invasive Surgery Center Jesús Usón (Cáceres, Spain).
CT was performed using a Philips BRILLIANCE CT-6, with a minimum slice thickness of 0.6 mm and a full-body speed of 0.75 seconds in 360°. OsiriX Lite software (v.11.0.3) was used to explore the CT-Dicom files, and render 3D models of the heart. For the endoscopic study of the heart, a flexible endoscope was used (Fujinon 200, EPX-2200 processor, endoscope EG250WR5: outer diameter 9.4 mm, length 110 cm).
After CT and endoscopy study, the preserved heart was shipped as a dry specimen back to Canada under CITES exportation (ES-LA-00003/17E) and CITES reimportation (17CA00017/CWHQ) permits. The heart, on arrival back to Canada, was still flexible in some areas, and full hardening occurred at ambient room temperature, while the heart was displayed on exhibition.
Despite advanced autolysis of the carcass noted at necropsy, the heart of L95 SRKW (O. orca) was successfully harvested, transported, and preserved by standard cold-temperature silicone plastination (Henry and Nel, 1993; de Jong and Henry, 2007; Henry et al., 2019). The final product was a moderately dilated and partially dissected beige specimen. Coronary artery filling with AC52 pigmented RVT silicone provided some contrasting color (Figs. 8-9).
External Morphology
The configuration of the external morphology (Figs. 8-10) shows a broadly conical shape and dorso-ventrally flattened aspect of the atrial and auricular surfaces, with narrow right and left margins. The atrial and auricular sides of the heart, the coronary sulcus, ascending aorta, pulmonary trunk and right and left auricles were identified and displayed.
Both coronary arteries had marked sinuosity. After dissection of these vessels, it was possible to see the right coronary artery arising from above the right semilunar valve of the aortic sinus. This artery had several parallel branches in the right portion of the coronary sulcus towards the subsinuosal interventricular sulcus, where the subsinuosal interventricular branch also divided into several vessels. The left coronary artery arose from the aortic sinus and projected a paraconal interventricular artery into the paraconal interventricular sulcus. A circumflex branch arose from the left coronary artery and ran caudally in the coronary sulcus towards the subsinuosal interventricular sulcus. Several parallel vessels were also observed in the left coronary artery.
Cardiac Computed Tomography
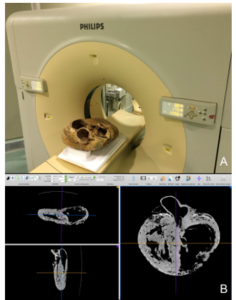
Figure 11. (A) Computerized tomography unit (Philips BRILLIANCE CT-6) used for plastinated heart exploration. (B) Reconstructed images of lateral-lateral and atrial-auricular. The sequence of images obtained in the major axis (dorso-ventral) allowed the remaining two planes to be reconstructed to identify the largest number of anatomical structures
Sequential imaging was performed along the dorso-ventral axis (Fig. 11a). The images obtained allowed the remaining two planes to be reconstructed to identify numerous anatomical structures (Fig. 11b). The level of radiopacity of the plastinated myocardium was sufficient to allow the identification of anatomical structures. A remarkable result found in the CT images was the presence of intramyocardial vascular caverns in both the interventricular septum and the walls of the ventricles. These structures did not appear to be pathological. In the CT sagittal plane that coincides with the axis of the ascending aorta (Fig. 12), the atria and ventricles were identified, and the left atrioventricular/mitral valve was seen. The greater thickness of the left ventricle (LV), as well as the relative capaciousness of the right ventricle (RV) was clearly appreciated. With greater magnification (Fig. 13), the valve of the aorta and the origin of the coronary arteries at the sinus of the aorta were identified. The radiopacity of the RTV silicone injected into the coronary arteries was readily apparent. In another sagittal plane, but displaced towards the auricular side (Fig. 14), the right atrioventricular or tricuspid valve was observed. In the same plane, a topographical septomarginal trabecula between the interventricular septum and the wall of the RV was noted. In a cross-sectional plane, the different conformation of the ventricular cavities was readily apparent (Fig. 15). A rounded apex was also observed in sagittal plane, with both the LV and RV contributing equally to the apical conformation (Figs. 13, 14).
The general morphology of the plastinated heart was well-preserved, as viewed by 3D rendering of the CT-Dicom files. An example is the clearly displayed atrial (Fig. 16a) and auricular sides (Fig 16b). Similarly, in oblique views, the left (Fig 16c) and right ventricles were clearly demarcated (Fig 16d).
Endoscopic Study
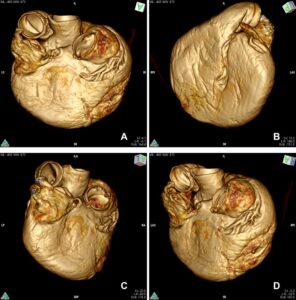
Figure 16. OsiriX Lite software (v.11.0.3) was used to explore the CT-Dicom files and render 3D models. The general morphology of the plastinated heart was preserved, so that the atrial (A) and auricular (B) sides were clearly displayed. Similarly, in oblique views, the left (C) and the right (D) ventricular borders can be seen
The RA and RV were initially explored via the following sequence: RA, right atrioventricular valve, RV, to the pulmonary trunk (Fig. 17a). In the RA, pectinate muscles projecting towards the auricula and part of the atrium wall were identified (Fig. 17b). By advancing the endoscope towards the RV, the right atrioventricular or tricuspid valve, with its three cusps and their attachment to the chordae tendineae, were observed in detail (Fig. 17c). Following the cords deep into the RV lumen, their attachment to the papillary muscles was observed (Fig 17d). The appearance of the RV wall trabeculae carneae (18b) was similar to that of the pectinate muscles of the RA. By magnifying the endoscopic image it was appreciated how the trabeculae appeared as small formations projecting into the lumen of the ventricle (Fig. 18a). Closer to the apex of the RV, a thick septomarginal trabecula was observed (Fig. 18b). After exceeding the RV limits, the pulmonary trunk was accessed. To best visualize the valve of the pulmonary trunk, a disto-proximal approach via the pulmonary vessel and towards the RV was used (Fig. 18c).
To explore the lumina of the LA and LV, entry was gained via the opening of the coalesced pulmonary veins of the LA, left atrioventricular valve, LV, and aorta. The LV had many of the same features of the RV. However, its smaller caliber allowed fewer structures to be observed than in the RV. In the first image with retroversion of the endoscope from the LV (Fig. 19a), the left atrioventricular valve was observed, as well as parts of the chordae tendineae and pectinate muscles of the left atrium. The endoscopic features of the LV wall (Fig. 19b) were similar to those observed in the RV, with numerous trabecular-like formations and endocardial bundles (Fig. 19c). From the LV it was not possible to pass through the sinus of the aorta as it contained the catheters used to inject silicone into the coronary arteries.
The plastinated killer whale heart was displayed during the 2017 ROM exhibition ‘Out of the Depths: The Blue Whale Story’ (Fig. 20). It will be subsequently displayed in related, upcoming exhibitions.
Plastination allows preservation of previously fixed, three-dimensional organs and, in this case, the heart of a rare Southern resident killer whale. The result is a specimen that preserves both the external and internal morphology.
Studies on marine mammal hearts are commonly based upon formalin-preserved, wet specimens. However, published descriptions of hearts preserved through plastination techniques are also available for relatively few species. These include the ringed seal (Phoca hispida) (Henry et al., 2005; Smodlaka et al., 2008), the bottlenose dolphin (Tursiops truncatus) (Contreras et al., 2015) and the blue whale (Balaenoptera musculus) (Miller et al., 2017). In some of these studies vascular color injection of the coronary arteries was used (Tarpley et al., 1997).
External Morphology
Regarding the external morphology of the heart of the killer whale, its flattened appearance was conspicuous, and has also been described in other marine mammals, such as the harbor porpoise (Rowlatt and Gaskin, 1975), minke whale (Ochrymowych and Lambertsen, 1984), beluga whale (Bisaillon et al., 1987), harp seal (Bisaillon, 1982), ringed seal (Smodlaka et al., 2008), bowhead whale (Tarpley et al., 1997), pygmy killer whale (Klomkleaw et al., 2005) and gray, sei and sperm whales (Truex et al., 1961). The two interventricular sulci, paraconal and subsinuosal, were well differentiated, as described in the minke whale (Rowlatt, 1981), the beluga (Bisaillon et al., 1987), and the pygmy killer whale (Klomkleaw et al., 2005). Paraconal and subsinuosal interventricular sulci appeared almost halfway between the cranial and caudal borders of the heart, and coursed directly to the apex (Klomkleaw et al., 2005).
The size of both coronary arteries was similar in the plastinated heart, and they had marked sinuosity (‘tortuosity’ of Rowlatt, 1981 and Truex et al., 1961) and similar contribution to myocardial vascularization. Similar morphology has been previously described in marine mammals, including the minke whale (Ochrymowych and Lambertsen, 1984), beluga whale (Bisaillon et al., 1988), bowhead whale (Tarpley et al., 1997), toothed whale (Rowlatt, 1981), and the pygmy killer whale (Klomkleaw et al., 2005), as well as sei and sperm whales (Truex et al., 1961). A contrasting condition of left or right coronary artery dominance was noted in the gray whale (left coronary artery) and in two of five sperm whales (right coronary artery) by Truex et al. (1961). However, equal size and distribution of both left and right coronary arteries was noted by Rowlatt (1981) for toothed whales and three of the five sperm whales investigated by Truex et al. (1961) Results observed in the pygmy killer whale coincide with the distribution described in this work, although Klomkleaw et al. (2005) mentioned a circumflex branch from the right coronary artery, a descriptive not recognized in the Nomina Anatomica Veterinaria. A circumflex branch was also described by Rowlatt (1981) for both minke and toothed whales. Anastomotic coronary arteries have been identified in a variety of cetacean species by several authors (Race et al., 1959; Truex et al., 1961; Ochrymowych and Lambertsen, 1984; Bisaillon et al., 1988; Tarpley et al., 1997), although none were identified in the present study.
Few reports of CT scans (Smodlaka et al., 2008; Ivančić et al., 2014) or MRI (Tarpley et al., 1997) for anatomic description of marine mammal hearts were found. No published reports of the use of endoscopy for examination of the lumen of the atria or ventricles in whales were located.
CT Study
The CT scan images facilitated study of the internal conformation of the killer whale heart. This approach has shown the distinctiveness of the ventricular walls, especially the relatively thin right ventricular wall, and reduction of the interventricular septum surface. This last has been related with the flattened heart shape and the thicker wall of the left ventricle (Rowlatt, 1981). A spacious right ventricle has been described in the harp seal (Bisaillon, 1982) and, with CT images, in the ringed seal (Smodlaka et al., 2008). Truex et al. (1961) noted ‘surprising’ thinning in the anterior and lateral ventricular walls, as well as the apex. Other authors described observing right ventricular hypertrophy in the minke whale heart, proposing that this condition could be related to hemodynamic changes associated with apneic diving and the accommodation of associated preload (Halina and Gaskin, 1978; Ochrymowych and Lambertsen, 1984).
The CT images of the plastinated killer whale heart showed that the apex was formed not only from the left ventricle, but also from the right ventricle, as has been described in the beluga whale (Bisaillon et al., 1987), harp seal (Bisaillon 1982), ringed seal (Smodlaka et al., 2008), sperm whale (Truex et al., 1961) and blue whale (Miller et al., 2017).
Endoscopy Study
Endoscopic exploration also facilitated detailed study of the valves in the plastinated killer whale heart. Previous references to the atrioventricular valves have been published in other marine mammals, such as the minke whale (Ochrymowych and Lambertsen, 1984). In ringed seals, the left atrioventricular valve resembles an interrupted circular valve without divisions into parietal and septal cusps (Smodlaka et al., 2008). However, we could readily identify each cusp of the atrioventricular valves. Moreover, the endoscopy study supplied a broad collection of detailed images of the inner anatomy of the atria and ventricles.
The occasion to plastinate a killer whale heart affords a unique opportunity to further inform the public and researchers about the adaptive variation in the cetacean heart, as well as offering novel educational moments. The plastinated and dissected SRKW heart was displayed during the Royal Ontario Museum’s 2017 exhibition ‘Out of the Depths, The Blue Whale Story’ and will be a focal asset in upcoming natural history exhibitions. The killer whale heart is one of a series of plastinated specimens used to inform and teach visitors about the unique character of the cetacean heart, as compared to the hearts of typical land mammals. This series includes the largest preserved vertebrate coronary specimen of a blue whale heart (Miller et al., 2017). The quality of anatomical detail has been excellent, amenable to both endoscopy and CT explorations. Overall, the project yielded insights into the complexities of large, hollow organ plastination, and the transfer of such a unique specimen across international borders during the various stages of its preparation. Like the preservation by plastination of a severely autolytic blue whale heart, the information derived from the SRKW project continues to improve the potential for conserving rare anatomical material in the future.
Bickley HC, Conner RS, Walker AN, Jackson RL. 1987: Preservation of tissue by silicone rubber impregnation. J Int Soc Plastination 1:30-39.
https://doi.org/10.56507/XVDP9663
Bigg MA, Olesiuk PF, Ellis GM, Ford JKB, Balcomb KC. 1990: Social organization and genealogy of resident killer whales (Orcinus orca) in the coastal waters of British Columbia and Washington State (SC/A88/ID39). In: Hammond PS, Mizroch SA, and Donovan GP editors. Individual Recognition of Cetaceans: Use of Photo-Identification and Other Techniques to Estimate Population Parameters. Rep Int Whal Comm Special Issue 12: 391-448.
Bisaillon A. 1982: Notes on the anatomy of the heart in the harp seal (Pagophilus groenlandicus Erxleben, 1777). Acta Anat (Basel) 114(2):177-84.
https://doi.org/10.1159/000145589
Bisaillon A, Martineau D, St-Pierre MA. 1987: Anatomy of the heart of the beluga whale (Delphinapterus leucas). J Morphol 191(1):89-100.
https://doi.org/10.1002/jmor.1051910109
Bisaillon A, Martineau D, St-Pierre MA, Béland P. 1988: Arterial and venous vasculature of the heart of the beluga whale (Delphinapterus leucas). J Morphol 195(3):305-12.
https://doi.org/10.1002/jmor.1051950305
Contreras VMD, Moreno CR, Sánchez FG. 2015. Descripción Anatómica de Cinco Órganos Internos del Delfín Nariz de Botella (Tursiops truncatus), a Través de la Técnica de Plastinación [Anatomy description of the five internal organs of the bottlenose dolphin (Tursiops truncatus) through plastinated technique]. Int J Morphol 33(2):571-579.
https://doi.org/10.4067/S0717-95022015000200026
COSEWIC. 2001: COSEWIC assessment and update status report on the Killer Whale Orcinus orca in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 47 p.
COSEWIC. 2008: COSEWIC assessment and update status report on the Killer Whale Orcinus orca, Southern Resident population, Northern Resident population, West Coast Transient population, Offshore population and Northwest Atlantic / Eastern Arctic population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. viii + 65 p.
Cozzi B, Huggenberger S, Oelscjläger H. 2017: Anatomy of Dolphins: Insight into Body Structure and Function. London: Academic Press, 438 p.
de Jong K, Henry, RW. 2007: Silicone plastination of biological tissue: cold-temperature technique Biodur S10/S15 technique and products. J Int Soc Plastination 22:2-14.
https://doi.org/10.56507/ZLMJ7068
Ford JKB. 2018: Killer whale (Orcinus orca). In: Würsig B, Thewissen JGM, Kovacs KM, editors. Encyclopedia of Marine Mammals 3rd ed. London: Academic Press, p 531-537.
https://doi.org/10.1016/B978-0-12-804327-1.00010-8
Gatesy, J, Geisler JH, Chang J, Buell C, Berta, Meredith RW, Springer MS, McGowen MR. 2013: A phylogenetic blueprint for a modern whale. Mol Phylogenet Evol 66(2):479-506.
https://doi.org/10.1016/j.ympev.2012.10.012
Halina WG, Gaskin DE. 1978: Functional anatomy of the coronary system of the harbour porpoise, Phocoena phocoena (L.) 1978. Can J Zool 56 (8)
https://doi.org/10.1139/z78-227
Henry RW. 1987: Plastination of an integral heart-lung specimen. J Int Soc Plastination 1(2):20-24.
https://doi.org/10.56507/KQKI2988
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7:27-31.
https://doi.org/10.56507/WUXP9436
Henry RW, von Hagens G, Seamans G. 2019: Cold temperature/Biodur. Anat Histol Embryol 48(6):532-538.
https://doi.org/10.1111/ahe.12472
Huggenberger S, Oelschläger H, Cozzi B. 2019: Atlas of the Anatomy of Dolphins and Whales. London: Academic Press, 519 p.
Ivančić, M, Solano M, Smith CR. 2014: Computed tomography and cross-sectional anatomy of the thorax of the live bottlenose dolphin (Tursiops truncatus). Anat Rec (Hoboken) 297(5):901-15.
https://doi.org/10.1002/ar.22900
Klomkleaw W, Viboonjan P, Chunsue N, Sailasuta A. 2005: Gross anatomy of the pygmy killer whale heart found in Thailand. Thai J Vet Med 35(2): 31-39.
https://doi.org/10.56808/2985-1130.2002
Miller JR, Henry RW, Nader P, Engstrom MD, Iliff S, Chereminskiy V, von Hagens G. 2017: The challenges of plastinating a blue whale (Balaenoptera musculus) heart. J Plast 29(2):22-29.
https://doi.org/10.56507/SJTL1096
National Marine Fisheries Service. 2008: Recovery Plan for Southern Resident Killer Whales (Orcinus orca). National Marine Fisheries Service, Northwest Region, Seattle, Washington.
Nomenclature, International Committee on Veterinary Gross Anatomical. 2012: Nomina Anatomica Veterinaria. 5th (revised version) edition. 5th Editorial Committee Hannover, Columbia, Gent, Sapporo: World Association of Veterinary Anatomists.
Ochrymowych C, Lambertsen RH. 1984: Anatomy and vasculature of a minke whale heart. Am J Anat 169(2):165-75.
https://doi.org/10.1002/aja.1001690205
Oostrom K. 1987a: Fixation of tissue for plastination: general principles. J Int Soc Plastination 1(1):3-11.
https://doi.org/10.56507/WLZH2223
Oostrom K. 1987b: Plastination of the heart. J Int Soc Plastination 1(2):12-19.
https://doi.org/10.56507/YWZL8112
Race, GJ, Edwards WL, Halden ER, Wilson HE, Luibel FJ. 1959: A large whale heart. Circulation 19 (6):928-32.
https://doi.org/10.1161/01.CIR.19.6.928
Raverty SA, Gaydos JK, St. Leger JA. 2014: Killer whale necropsy and disease testing protocol. NOAA publication
Rowlatt U, 1981: The cardiac ventricles of a baleen whale (Balaenoptera acutorostrata: Minke whale) and a toothed whale (Hyperoodon ampullatus: Bottlenose whale). J Morphol 168(1):85-96.
https://doi.org/10.1002/jmor.1051680109
Rowlatt U, Gaskin DE. 1975: Functional anatomy of the heart of the harbor porpoise, Phocaena phocaena. J Morphol 146(4):479-93.
https://doi.org/10.1002/jmor.1051460405
Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee. 2008: p 42-43, Guideline for disinfection and sterilization in healthcare facilities. Publication of Centers for Disease Control and Prevention, USA.
Smodlaka, H, Henry RW, Schumacher J, Reed RB. 2008: Macroscopic anatomy of the heart of the ringed seal (Phoca hispida). Anat Histol Embryol 37(1):30-5.
https://doi.org/10.1111/j.1439-0264.2007.00791.x
Tarpley, RJ, Hillmann DJ, Henk WG, George JC. 1997: Observations on the external morphology and vasculature of a fetal heart of the bowhead whale, Balaena mysticetus. Anat Rec 247(4):556-81.
https://doi.org/10.1002/(SICI)1097-0185(199704)247:4<556::AID-AR14>3.0.CO;2-O
Tiedemann K, von Hagens G. 1982: The technique of heart plastination. Anat Rec 204(3):295-299.
https://doi.org/10.1002/ar.1092040315
Truex, RC, Nolan FG, Schneider HP, Perlmutter HI. 1961: Anatomy and pathology of the whale heart with special reference to the coronary circulation. Anat Rec 141:325-53.
https://doi.org/10.1002/ar.1091410408
Von Hagens G. 1986: Heidelberg Plastination Folder: Collections of technical leaflets for plastination. Rathausstrasse 18, Heidelberg, 691626: Biodur Products.
Von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4):411-421.
https://doi.org/10.1007/BF00309677