1Department of Morphology, Federal University of Espirito Santo, Brazil
2Biochemistry and Pharmacology Graduation Program, Federal University of Espirito Santo, Brazil
3Department of Physiology Science, Federal University of Espirito Santo, Brazil
4Department of Medical Education, College of Medicine and Life Sciences, University of Toledo, Ohio USA
The recent creation of plastination labs in Brazil and the increased demand to convert existing glycerin-fixed specimen collections to plastinated ones, prompted this study to evaluate the viability of converting previously glycerin-fixed specimens to plastinated specimens. Four human specimens (a lower limb segment, a lung, a fetal liver and a human femur), and one bovine kidney were used for this research. A variation of the Biodur S10 technique for plastination, at room temperature was used for this experiment. Previously glycerin-fixed specimens were subjected to five successive baths of acetone in increasing concentrations (80, 90, 95, 97, and 100% volume/volume) at room temperature (≅ 25ºC). After dehydration. The specimens were submerged in a mixture composed of silicone Biodur® S10 and the crosslinker Biodur S6®, respectively in the proportion of 92:8 mass/mass at room temperature. A progressively increased vacuum was applied, maintaining a slow bubble pattern with approximately one bubble/second. The impregnation lasted seven days. After impregnation, the specimens were exposed to the catalyst Biodur® S3 for hardening. The macroscopic aspects of the specimens were evaluated, comparing them before (in glycerin) and after plastination. Plastination of glycerin-fixed specimens has been proved possible and produced adequate results, preserving morphoanatomical aspects, color, and dimensions of the specimens. Furthermore, it is possible to use only acetone to remove glycerin in the plastination method. The degree of the milky aspect of the acetone, which is caused by the presence of glycerin, is the parameter to determine the efficiency of the exchange of glycerin by acetone.
glycerin; glycerin-fixation; plastination; Giacomini method
Athelson S Bittencourt, Federal University of Espirito Santo, Health Sciences Center, Marechal Campos Avenue, 1468 Maruipe Vitoria - ES, Brazil, 29.043-900, Fax: +55 27 33357358, athelson@hotmail.com
![]()



The search for techniques for preservation of biological tissues goes back more than five thousand years, where, for religious or scientific reasons, chemical or physical means were used to preserve specimens or bodies. From the Egyptians to the present day, several techniques have been developed for this purpose (Paula, 2014). Formalin-fixation is one of the most commonly used methods for preservation and conservation of biological tissues (Coelho, 2009; Paula, 2014; Karam et al., 2016). This wide use is due to its low cost, and its rapid tissue penetration and fixation. However, it has disadvantages, like its strong and irritating odor and the fact of being highly harmful to health (Silva et al., 2016). In 2006, the International Cancer Research Agency (IARC) proved the carcinogenic nature of formaldehyde on human cells. For these reasons, there is an increasing tendency to substitute formaldehyde for other cadaveric conservation methods (Paula, 2014; Karam et al., 2016; Silva et al., 2016).
Glycerin, or glycerol, was discovered in 1762 by the chemist Karl Sheele, and later used by the Italian anatomist and neuroscientist Carlo Giacomini from the University of Turin, Italy, in anatomical techniques (Gigek et al., 2009). Glycerin has the capacity of inducing tissue dehydration, an effect responsible for its antiseptic characteristic. Glycerin-fixation, originally known as the Giacomini method, has undergone some changes over time, however, it follows fundamental steps: fixation, clearing, dehydration, and impregnation with glycerin (Neto and Bigoni, 2014). This technique allows the production of non-toxic specimens, lighter and without the need for maintenance in preservative solutions. The disadvantages of using glycerin as a preservation tool are: (1) the progressive darkening of tissues over time, (2) glycerin oozing from the specimens due to room temperature variations, (3) need of continuous maintenance for whitening and re-glycerination of the tissues overtime, and (4) tissue shrinkage (Silva et al., 2011; Cury, 2012; Paula, 2014).
Many universities in Brazil use the glycerin-fixation technique to maintain their specimen collections. A survey carried out in 2015 showed that 56.4% of the 81 brazilian medical schools surveyed in the study use the glycerin-fixation technique to preserve anatomical specimens, and only 2.6% use the technique in plastination (Silva et al., 2016). The recent creation of plastination labs in Brazil, the increased demand to convert existing glycerin-fixed specimen collections to plastinated ones, and the shortage of studies in the literature related to the plastination of previously glycerin-fixed specimens prompted this study. The objective of this work was to evaluate the outcome of previously glycerin-fixed specimens after plastination, using only acetone to remove glycerin during the process.
Four human specimens (a lower limb segment, a lung, a fetal liver, and a fetal femur), and one bovine kidney, all of which had been previously glycerin-fixed for about 3 years, were used in this experiment. All the specimens belong to the collection of the anatomy laboratory of Federal University of Espirito Santo, Brazil. The human lower limb had been previously dissected to show anatomical structures of interest, and the skin and subcutaneous tissues were removed to expose the knee joint and its components. The human anatomical specimens were provided by the collection of the Department of Anatomy of the Federal University of Espírito Santo (UFES) and have all documentation of receipt of unclaimed body regularized, according to Federal Law No. 8,501 (November 30, 1992) which authorizes their use for teaching and research purposes. The use of bovine kidney was approved by the Animal Ethics Committee of UFES, registered under No. 31/2019.
A variation of the Biodur S10 technique of the protocol for plastination, at room temperature (Starchik and Henry, 2019), was carried out: the previously glycerin-fixed specimens were subjected to five successive baths of acetone in increasing concentrations (80, 90, 95, 97, and 100% v/v), for 7 days each at room temperature (≅ 25ºC). With the aid of an acetonometer, the concentration of the acetone was recorded, and it was considered glycerin-free when the acetone bath was above 99% volume/volume (v/v) purity after 7 days of the last bath. Although the anatomical specimens were dehydrated during the glycerin-fixation process, the acetone baths served to remove glycerin from the biological tissues and served as a volatile intermediate solvent for replacement by silicone. After this stage, the specimens were submerged in a mixture composed of silicone Biodur® S10 and the crosslinker Biodur® S6, in the proportion of 92: 8 m/m, respectively, in a vacuum chamber at room temperature (≅ 25ºC). A progressively increased vacuum was applied. The vacuum was adjusted two to three times a day, until a slow bubble pattern of approximately one bubble/second was achieved (de Jong and Henry, 2007; Raoof et al., 2007). The impregnation lasted seven days. The vacuum should not be <10 mmHg, since the vapor pressure of cross‐linker/S6 is near 10 mmHg (Starchik and Henry, 2019). After the vacuum pump was turned off, the specimens remained immersed in the silicone for a further 24-hour period. They were then removed from the chamber, and the excess polymer was drained for three to four days. After draining, the specimens were brushed with the catalyst Biodur® S3 for hardening at room temperature. The macroscopic aspects of the specimens were evaluated, comparing them before (in glycerin), and after plastination.
Five baths of room temperature acetone (80, 90, 95, 97, and 100% v/v) proved to be adequate and efficient to remove the glycerin present in the specimen (tissue). However, during each bath the acetone became progressively milkier over time. This was due to contamination of the acetone by the glycerin being extracted from the specimens. Nonetheless, with the progressive removal of glycerin by diffusion with each replacement of acetone, the new acetone became less milky than the previous one. At the end of the last bath, no turbidity was observed. The contaminated acetone (glycerin+acetone+water) was recycled in a distiller for purification and later use.
Seven days of forced impregnation at room temperature proved to be sufficient for replacement of acetone with silicone. The frequency of bubbles of acetone emerging from the silicone and bursting on the surface of the polymer was used as a parameter to dictate the speed of the impregnation. A pattern of 1 bubble/second was a parameter used in order to avoid greater tissue shrinkage. Although no measurements of shrinkage were taken, there was no noticeable visual shrinkage in the specimens and their anatomical structures. The curing step took 3 days, and did not require re-application of the catalyst. After the plastination process was completed, the specimens were dry and odorless, and maintained their previous morphoanatomical characteristics. There were no indications of glycerin, or changes in color or shape. These specimens have been stable, with no silicone or glycerin seepage, for the last two years. Figures 1 to 5 show glycerin-fixed specimens before and after the plastination process.
 Figure 1: Comparison of a human lung preserved in glycerin (A) and after plastination (B). |
 Figure 2: Comparison of a human femur preserved in glycerin (A) and after plastination (B). |
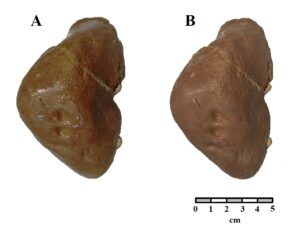 Figure 3: Comparison of a human fetal liver preserved in glycerin (A) and after plastination (B). |
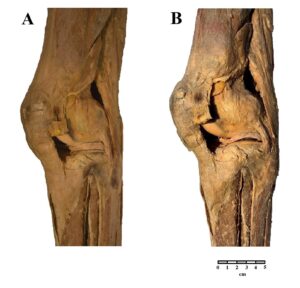 Figure 4: Comparison of a human lower limb preserved in glycerin (A) and after plastination (B). |
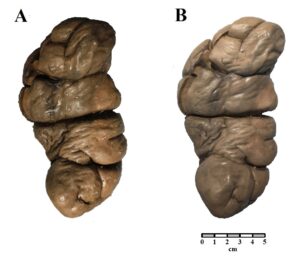 Figure 5: Comparison of a bovine kidney preserved in glycerin (A) and after plastination (B). |
Plastination has benefits in comparison to wet specimens (either formalin- or glycerin-fixed) since this preservation technique provides more durable, odorless, tactile, and non-toxic specimens. In addition, when completely plastinated with silicone, specimens do not drain indefinitely, and there are no reports in the literature of color changes over time (von Hagens et al., 1987). Pádua et al. (2016) proposed the chemical reversal of glycerin as a step prior to plastination, as follows: the glycerin-impregnated specimen was immersed in 50% alcohol for seven days, washed for 24 hours in running water, immersed in potassium hydroxide solution 1.5% for eight hours, washed in running water for 48 hours, and immersed in 70% alcoholic solution for another 72 hours. After these steps, the specimens go on to dehydration. In the research reported here, we sought to verify if it is possible to efficiently remove glycerin from specimens using only acetone.
The acetonometer is an analytical instrument that measures the concentration of acetone through its density. Thus, considering that the density of acetone (0.784 g / cm³) is different from that of water (0.997 g / cm³) and glycerin (1.26 g / cm³) (Solomons and Fruhle, 2001), the acetonometer was a suitable instrument to monitor the removal of glycerin and residual water from biological tissues used in this work.
Although the conservation principle of the glycerin-fixation technique is dehydration, glycerin is not a volatile solvent and, therefore, the specimens were subjected to dehydration in acetone. Therefore, the acetone served as an intermediate solvent to be replaced by silicone. During dehydration, the exchange of substances between biological tissues and the solvent (acetone) occurs by simple diffusion. The choice of a greater volume and the number of replacements of acetone baths was made to guarantee a more efficient removal of glycerol from the specimens, since we did not know its behavior when exposed to acetone. The substitution of glycerin by acetone was evidenced by the change in transparency of the acetone bath. The more glycerin diffused into the acetone, the milkier it became. After the last acetone bath, the milky aspect was no longer perceived, probably due to a complete, or almost complete, removal of glycerin. The use of hydrated acetone may have helped in the removal of glycerin, since the solubility of glycerin in water is greater than in alcohol and acetone.
It is known that the glycerin-fixation process causes a certain amount of tissue shrinkage, which is mainly caused by the dehydration promoted by alcohol at room temperature (Brown et al., 2002). The impregnation rate is an important factor in the degree of tissue shrinkage, as even at room temperature (20 °C), the S10 silicone viscosity remains substantially higher (460 mPa.s) than acetone viscosity (0.33 mPa. s), which determines some level of tissue retraction. Therefore, we adopted a slower pace of vacuum progression (1 bubble/second/observed area), allowing the acetone to be extracted from the specimens slowly, permitting a more efficient substitution of the acetone by the silicone, and thus avoiding greater shrinkage during impregnation (Monteiro et al., 2018).
One of the advantages of using plastinated specimens versus the glycerin-fixed specimens is that plastinated specimens are dry in comparison to the constant wet feeling of the glycerin-fixed specimens, with the latter requiring the use of gloves during handling of the specimens.
In the qualitative macroscopic analysis of the specimens regarding color, volume, and texture aspects, the results obtained in this study were extremely satisfactory, since the parameters analyzed did not show significant changes. The impregnation and curing phases of the plastination process appear to have proceeded normally in previously glycerin-fixed specimens. These results are different from a few authors, who mentioned that all embalming fluids containing long chain alcohols (e.g., glycerol) have to be removed before dehydration (Fasel, 1988; Ravi and Bhat, 2011; Sora, 2016;), and Padua et al. (2016), who described several chemicals (including ethanol) to remove the glycerin from glycerin-fixed specimens before plastination. Although the solubility of glycerin is greater in ethanol than in acetone (Clasen et al., 2015; Marçal, 2015), the results of this research demonstrate that it is possible to plastinate specimens without a pre-treatment to remove glycerin. Acetone is also an organic solvent soluble in water and glycerin, permitting the removal of these substances efficiently without the need for other chemicals. The technique proposed by our study saved time and eliminated the cost of other chemical reagents, which are not necessary using the protocol described here.
In order to investigate a method of plastination for glycerin-fixed specimens, plastination at room temperature was chosen, because higher temperatures facilitate the replacement of glycerin by acetone in dehydration, and acetone by silicone in forced impregnation, when compared to the cold temperature method. This is directly related to the substances' viscosities, since at room temperature the viscosities are lower and, thus, the fluid replacement dynamics are easier, faster, and more efficient. At negative temperatures, glycerin becomes more viscous, which can affect its replacement by acetone. In this sense, according to chemistry, in general, the miscibility of substances tends to decrease at lower temperatures. In addition, impregnation at room temperature causes less tissue shrinkage, considering that the specimen has already suffered a certain degree of shrinkage in the glycerin method.
Some limitations of the method should be mentioned: (1) given the miscibility of glycerin in acetone, the use of larger volumes and quantities of acetone baths is suggested for more efficient removal of glycerin; (2) the proposed plastination method has been tested only at room temperature; (3) the acetone used in dehydrations must be recycled/distilled after use to avoid contamination of other specimens.
Plastination of glycerin-fixed specimens is possible and produces adequate results, which preserve the morphoanatomical aspects, color, and dimensions of the specimens. From the results of this research, it can be verified that acetone may be the only chemical product necessary to remove glycerin from the samples, producing final specimens without apparent residue of glycerin, and of good quality.
Acknowledgments
The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Brown MA, Reed RB, Henry RW. 2002: Effects of dehydration mediums and temperature on total dehydration time and tissue shrinkage. J Int Soc Plastination 17: 28-33.
https://doi.org/10.56507/XNQM4606
Clasen CD, Lisboa MT, Pinto AMTP, Ribeiro AS, Vieira MA. 2015: Avaliação de métodos de solubilização para determinação de metais em glicerina proveniente da produção de biodiesel por técnicas de espectrometria atômica [Evaluation of solubilization methods for the determination of metals in glycerin from biodiesel production by atomic spectrometry techniques]. Quim Nova 38 (1): 77-84 [in Portugese].
Coelho M. 2009: O formaldeído em ambiente laboral: determinação do ácido fórmico em urina de trabalhadores de uma fabrica produtora de formaldeído [Formaldehyde in the workplace: determination of formic acid in the urine of workers in a formaldehyde-producing factory (free translation)]. Master's thesis in Analytical, Clinical and Forensic Toxicology, Porto University, Portugal [in Portuguese].
Cury FS. 2012: Elaboração laboratorial padrão em anatomia animal e técnicas anatômicas [Standard laboratory design in animal anatomy and anatomical techniques (free translation)]. Master's thesis in Anatomy of Domestic and Wild Animals. University of São Paulo [in Portuguese].
de Jong, K, Henry RW. 2007: Silicone plastination of biological tissue: Cold-temperature technique Biodur S10/S15 technique and products. J Int Soc Plastination 22: 2-14.
https://doi.org/10.56507/ZLMJ7068
Fasel JHD. 1988: Use of plastinated specimens in surgical education and clinical practice. Clin Anat 1(3): 197-203.
https://doi.org/10.1002/ca.980010306
Gigek T, Oliveira JEM, Neto ACA, Carvalho WL, Pereira FV, Almeida AH. 2009: Estudo Analítico da Técnica de Glicerinação Empregada para Conservação de Peças Anatômicas de Bovinos [Analytical study of the glycerination technique used for the conservation of anatomical parts of cattle]. In: UNESP SCIENCE SYMPOSIUM, V., 2009.
Karam RG, Cury FS, Ambrosio CE, Mançanares CAF. 2016: Uso da glicerina para a substituição do formaldeído na conservação de peças anatômicas [Glycerin can replace formaldehyde for anatomic conservation]. Pesq Vet Bras 36(7):671-675 [in Portugese].
https://doi.org/10.1590/S0100-736X2016000700019
Marçal MS. 2015: Valorização da glicerina [Valuing glycerin]. Dissertation for obtaining a Master's degree in Chemical Engineering. Lisboa, Portugal [in Portuguese].
Monteiro YF, Juvenato LS, Bittencourt ASV, Siqueira BMM, Monteiro FC, Baptista CAC, Bittencourt AS. 2018: Influence of the temperature on the viscosity of different types of silicone. J Plast 30 (1): 4-9.
https://doi.org/10.56507/HETT9088
Neto RAF, Bigoni PS. 2014: Substituição do formaldeído pela glicerina na conservação de preparações anatômicas [Replacement of formaldehyde in the conservation of glycerine anatomical preparations]. R Laborativa 3 (3): 75-87. http://ojs.unesp.br/index. php/rlaborativa [in Portugese]
Padua AC, Carvalho P, Godoy JRP. 2016: Técnica de reversão do método de glicerinação e aplicação da técnica de plastinação com silicone nacional Polisil Silicones - Poliplast 40 [Reversal technique of the glycerination method and application of the plastination technique with national silicone Polisil Silicones - Poliplast 40 (free translation)]. In: XXVII Brazilian Anatomy Congress, 2016 [in Portugese].
Paula R. 2014: Análise morfológica da propriedade de compostos vegetais na conservação de tecidos cadavéricos [Morphological analysis of the property of plant compounds in the conservation of cadaveric tissues (free translation)]. Doctoral Thesis in Domestic and Wild Animal Anatomy, Faculty of Veterinary Medicine and Animal Science, University of São Paulo [in Portuguese].
Raoof A, Henry RW, Reed RB. 2007: Silicone plastination of biological tissue: room-temperature technique - Dow/Corcoran technique and products. J Int Soc Plastination 22: 21-25.
https://doi.org/10.56507/AWAC9285
Ravi SB, Bhat VM. 2011: Plastination: A novel, innovative teaching adjunct in oral pathology. J Oral Maxillofac Pathol 15(2): 133-137.
https://doi.org/10.4103/0973-029X.84475
Silva GR, Cortez POB, Lopes ISL, Teixeira BACB, Leal NMS. 2016: Métodos de conservação de cadáveres humanos utilizados nas faculdades de medicina do Brasil [Methods of conservation of human cadavers used in medical schools in Brazil]. Rev Med (São Paulo) 95(4): 156-161 [in Portugese]
https://doi.org/10.11606/issn.1679-9836.v95i4p156-161
Silva NA, Galvão APO, Fraga KB, Oliveira RG, Barbosa RF, Campina RCF, Santos TR, Magalhães CP. 2011: Comparative study between two techniques using glycerin in the conservation of a central nervous system. J Morphol Sci 28 (4): 280-282.
Solomons TWG, Fruhle CB. 2001: Solomons' Organic Chemistry, 7th ed, John Wiley & Sons Publishing Company S. A., Washington, p. 76-77.
Sora C-M. 2016: The general protocol for the S10 technique. Res Clin Med 1 (1): 14-18. https://www.resclinmed.eu/public/data_files/articles/12/article_12.pdf
Starchik D, Henry RW. 2019: Room temperature/Corcoran/Dow Corning™-Silicone plastination process. Anat Histol Embryol. 48: 539-546 https://doi.org/10.1111/ahe.12505
https://doi.org/10.1111/ahe.12505
von Hagens, G; Tiedemann, K; Kriz, W. 1987: The current potential of plastination. Anat Embryol Histol 157: 411-421.
https://doi.org/10.1007/BF00309677