Department of Anatomy, Physiological Sciences, and Radiology North Carolina State University College of Veterinary Medicine Raleigh, NC 27606, USA
In anatomy laboratories, many mechanical problems are encountered by students and instructors while handling and studying large animal gastrointestinal tracts, especially adult ruminant stomachs. Because of organ weight and flaccidity of the organ wall, it is often difficult to demonstrate normal topographical relations of these organs in suspended cadavers. When the tracts are removed from the abdominal cavity and emptied of ingesta, almost all reference points are lost for students attempting to study the internal structure of these hollow organs and their position in the cadaver.
Silicone; Dilation; Biodur; S10
S. D. Holladay Department of Anatomy, Physiological Sciences, and Radiology North Carolina State University College of Veterinary Medicine Raleigh, NC 27606, USA
![]()



In anatomy laboratories, many mechanical problems are encountered by students and instructors while handling and studying large animal gastrointestinal tracts, especially adult ruminant stomachs. Because of organ weight and flaccidity of the organ wall, it is often difficult to demonstrate normal topographical relations of these organs in suspended cadavers. When the tracts are removed from the abdominal cavity and emptied of ingesta, almost all reference points are lost for students attempting to study the internal structure of these hollow organs and their position in the cadaver.
A number of techniques have been developed for producing rigid models of hollow gastrointestinal organs that can be handled by students and compared with the embalmed tracts. At first, fresh organs were removed from euthanatized animals, emptied, dehydrated in alcohol, and then inflated and placed on an air line to dry (Church, 1968). This method produced a rigid but very fragile model. To improve durability, fiberglass resin and mats or other rigidly drying resins were applied to the external surfaces of air-dried specimens (Church, 1968; Kitchell et al., 1961). These fiberglass- reinforced models are reasonably resistant to everyday use in anatomy labs, but will shatter if dropped or roughly handled, and are difficult to repair. Also, small species of dermestid beetles rapidly damage or destroy such preparations when insecticides are not used regularly.
This report describes a technique for plastination of large animal hollow gastrointestinal organs in a life-like, inflated position.
Gastrointestinal viscera were taken from recently euthanized animals and flushed with water until all contents were removed. Some specimens were submerged overnight at +5°C in a low formaldehyde (Klotz) fixative solution. Other specimens were not exposed to any preserving chemicals, but instead placed directly into cold (-20°C) acetone after flushing.
Freeze substitution dehydration of the viscera required about one week to complete. The viscera were filled with acetone by using a funnel and pouring acetone directly into the organ. Viscera were turned over every day to prevent trapped air bubbles from impeding dehydration of inner surfaces. After three to four days, the viscera were transferred to fresh (100%) acetone. Due to the low actual tissue volume of the hollow organs, along with the high acetone: tissue ratio of the submerged, filled viscera, this second change was usually all that was required to achieve sufficient freeze substitution of the organs.
Once acetone dehydration was complete, the organs were removed and the acetone was drained. To allow the visceral lumens to fill with S-10 silicone under vacuum, several holes were made through the organ walls using a 16 gauge needle. The organs were weighted down to prevent an initial tendency to float in the silicone. After several days under vacuum, visceral lumens had filled with silicone and floating was no longer noticed. Impregnation of organ walls with silicone was carried out by gradual decrease in vacuum to 5 microns, over a period of ten days to two weeks.
After impregnation was complete, viscera were allowed to drain on a tray in the plastination unit. Draining of the hollow organs was allowed to continue for several hours, and initially was enhanced by having the vacuum pump turned on and running at maximum level (air value closed). Once the organs were removed from the vacuum chamber, the remaining silicone was manually expressed and returned to the plastination unit. To allow excess silicone to drain from the specimen, the organs were suspended over a bucket at room temperature overnight.
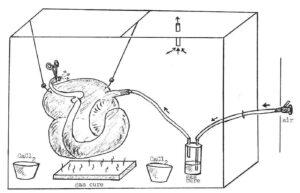
Figure 1. Schematic for gas curing large specimens. Air is bubbled through Sg to inflate the organ and cure the inner surface. Gas cure is also placed in a low pan to cure the exterior of the organ.
Curing of the viscera was accomplished under a hood in a large plastic bag that was pulled around a frame made of 2" x 2" lumber (Fig. 1). The organ was connected to an air line, with a heavy-walled plastic bottle containing gas cure interposed between the specimen and air jet. Additional containers of CaCl2 powder and gas cure were placed into the curing chamber. The air jet was turned on, and the extent of organ inflation controlled by hemostats clamped on the distal end (duodenum, transverse colon, etc.) of the organ. To prevent excess silicone from pooling and hardening inside the viscera, holes were made at the lowest point of each chamber with a 14 gauge needle. The specimen was allowed to cure overnight, then removed and windows cut with a scalpel for viewing of interior surfaces. After curing, specimens were placed overnight on a table in front of an air intake vent.
Plastinated gastrointestinal viscera (Figs 2-5), produced by the present technique, have been well received by faculty and students and have replaced the formerly used fiberglass models in our laboratory. Viscera prepared without fixation were more reddish-brown in color than those lightly fixed, but otherwise not noticeably different. Many of the viscera were too large for our conventional curing chamber, making the described plastic container necessary. We have found that such plastic bags are inexpensive ($0.80 for a 36" x 48" bag) and serve as a functional chamber, capable of being used with almost any size specimen. We have cured specimens up to seven feet long in plastic bags.
With the interior of inflated viscera being a positive pressure area, excess silicone is rapidly blown out of the organs through the small holes made in downward areas. Blowing gas cure through viscera cures the inner surface, while the gas cure saturated air exiting the viscera, along with gas cure from the container in the chamber, cures the exterior of the viscera. Curing is rapid by this method, occurring overnight. Finally, placing cured organs in front of a high air movement intake vent quickly removes excess gas cure, thus eliminating the formation of white precipitate (moisture reacting with gas cure) on the surface of plastinated specimens in our laboratory.
Church, DC: A simple method for preserving the ruminant stomach. J Anim Sci 27:1525-1526, 1968.
https://doi.org/10.2527/jas1968.2761525x
Kitchell, RL, J Turnbull, RA Nordine, SC Edgell: Fiberglass technique of preparation of natural models off the ruminant stomach. J Am Vet Med Assoc 138:329-331, 1961.