University of Torino, Department of Human Anatomy and Physiology, C.so M. D' Azeglio 52, Torino, ITALY
This paper presents an alternative approach to the study of gross anatomy which may be pursued in Medical Schools using plastination techniques. Specimens fixed with formalin and alcohol-based techniques were utilized as the source of specimens to be plastinated; the reason for this choice was a result of the decreased organ availability in Italy due to religion and restrictive laws. The procedure for preparation of the plastinated specimens is presented. This particular approach may be considered by Medical Schools where teaching sets are restricted due to a scarcity of organ donors or in the presence of a restrictive law.
Formaldehyde; Silicone; Plastination;
Mario Cannas University of Torino, Department of Human Anatomy and Physiology, C.so M. D' Azeglio 52, Torino, ITALY
![]()



This paper presents a supplement and alternative for the study of gross anatomy, which may be pursued in Medical Schools using the plastination technique. To the known advantages of this technique (ease of handling, good preservation, formaldehyde free specimens with the possibility to expand these techniques to other research fields like paleopathology and forensic medicine) is added the possibility of using old formalin- fixed specimens as a source of material when a scarcity of organs, for various reasons, hinders the teaching process. This choice of specimens results from the decreased organ and body supply in Torino and of all Italy; even if an Organ Donation Program was organized, the scarcity of organs does not assure availability of human material in the future. The Italian law provides for organs to be used for transplantation into living subjects, but does not provide for other scientific or didactic uses without consent.
Religious reasons also make it difficult to obtain teaching specimens. However, at the University of Torino Medical School, we have a large collection of formalin and alcohol-fixed specimens from anatomical dissections which were used in the gross anatomy laboratory in past years. Many specimens had been used very little, possibly because fixation resulted in wet slippery tissues, requiring the students to wear gloves to protect themselves from the fixative. Another consideration is the carcinogenic potential of formaldehyde. This paper deals with specimens treated with formalin and alcohol-based fixatives, which were used in the past years, and currently have been utilized as the source for specimens to plastinate.
Anatomical specimens were plastinated using modifications of the standardized plastination process (Tiedemann & von Hagens, 1982; von Hagens et al., 1987), especially with regard to the treatment times, which were generally lengthened. The resultant plastinated specimens included: heart, forearm, kidneys, spleen, entire brain and hemispheres.
FIXATION:
The specimens were all fixed and stored in a formaldehyde solution for several years. The exact duration of storage for many organs (spleen, heart, kidney and sectioned kidney) and concentration of formalin and alcohol solutions are unknown. However, the brain and the cerebral hemispheres were fixed in alcohol and stored in formalin for more than 10 years. The hand and the forearm were stored in formalin for the last 10 years. In most cases the principle of buffered fixation was not likely followed. The samples were stored at room temperature and used in the anatomic theater for teaching and in the museum for record.
DEHYDRATION:
Before starting the dehydration process, samples were washed in tap water for 24 hours, to rinse out the excess fixative. Blood inside the arteries and veins was not removed. The samples were pre-cooled overnight at 4°C. Freeze substitution dehydration was performed, using cold acetone with a fluid/specimen ratio of 10:1. The acetone was changed three times; except, the brain and hemispheres which had five changes to obtain a more lipid-free acetone. When all specimens were 100% dehydrated, they were placed in room temperature acetone for one week to complete defatting.
IMPREGNATION:
Standard forced impregnation of a Biodur S10-S3 mixture was used for all specimens at -25 °C. The vacuum was created after 48 hours of specimen-polymer equilibration and increased slowly over a four week interval. Impregnation was considered complete when bubbles were no longer viewed, resulting in a four to eight week impregnation time.
CURING:
After excess resin was allowed to drain from the specimens, they remained at room temperature for 6 weeks and at 40 °C for two weeks. During this time, the specimens were periodically rotated to avoid superficial deformities. After this time, the specimens were placed in a chamber on a metallic grid and exposed to Biodur S6 to enhance polymerization. After three to four weeks, the specimens were removed from the gas cure chamber and they were placed in a dry place for four weeks at room temperature with calcium chloride present the last two weeks.
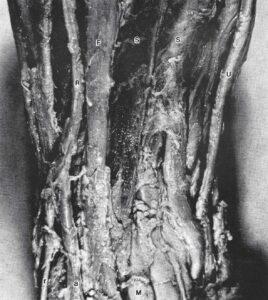
Figure 1. Plastinated forearm. Superficial digital flexor muscle (S), Flexor carpi radialis muscle (F), Dinar artery and nerve (U), Radial artery and nerve (R), Superficial branch of radial nerve (r), Radial artery (a) turning into the anatomical snuff box, Median nerve (M).
The use of plastinated specimens for teaching anatomy to students, residents and clinicians is well known (Holladay and Hudson, 1989). Relationship of anatomical structures can be examined, as well as, the identification of different tissue layers, nerves and vessels. (Baptista et al., 1989).
Anatomical relationships of the vessels, nerves and muscles are demonstrated in the forearm preparation (Fig. 1). The old formalin-fixed brain and hemisphere (Figs. 2, 3) resulted in a final color close to the color of the brains prior to plastination. Starting with high quality formalin-fixed brains, which had been air-stored and displayed in our museum (Fig. 4), the plastinated process produced little shrinkage.
Polymerizing resins, used as embedding media for tissues and organs, introduced in 1949, produce minimal distortion (Bennet et al., 1976). Many of these polymers were introduced to be used in electron microscopy or for enzymatic histochemistry at the light microscopic level (Wolfe, 1956; Anker et al., 1974). Our next step will be to use the Biodur E 12 technique, that has proved to be an excellent embedding medium for histology for various tissues. We will examine the implications for possible usage in the field of the paleopathology and forensic medicine. Naturally the preservation of histological architecture would depend upon the choice of fixative used. Another project will be to deplastinate these long- term fixed and plastinated specimens and prepare them for histological examination to evaluate tissue quality.
Utilizing long-term fixed specimens for plastination, resulted in good preservation of specimens for teaching and observation. The structural integrity of the specimens remained sound and aesthetically pleasing. We suggest this source of specimens as an appropriate way to supplement Medical School teaching sets if organ donors are scarce, or as in our case, in the presence of a restrictive law.
ACKNOWLEDGEMENTS: The authors thank the Department of Human Anatomy and Physiology of the University of Torino and support by the Ministerial Grant (60%).
Anker G, K Scheers-Dubbeldam, C Noorlander: An epoxy resin embedding technique for large objects. Stain Technol 49:183-188,1974.
https://doi.org/10.3109/10520297409116974
Baptista CAC, M Skie, RA Yeasting, N Ebraheim, WT Jackson: Plastination of the wrist: Potential uses in education and clinical medicine. J Int Soc Plastination 3:18-21,1989.
https://doi.org/10.56507/XENF9035
Bennet HS, AD Wyrick, SW Lee, JH McNeil: Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol 51:71-97, 1976.
https://doi.org/10.3109/10520297609116677
Holladay SD, L Hudson: Use of plastinated brains in teaching neuroanatomy at the North Carolina State University, College of Veterinary Medicine. J Int Soc Plastination 3:15-17, 1989.
https://doi.org/10.56507/FEKB4686
Tiedemann K, G von Hagens: The technique of heart plastination. Anat Rec 204:295-299, 1982.
https://doi.org/10.1002/ar.1092040315
von Hagens G, K Tiedemann, W Kriz: The current potential of plastination. Anat Embryol 175:411-421,1987.
https://doi.org/10.1007/BF00309677
Wolfe, K: Plastic-embedded hearts - Cleared and corroded specimens. Arch Pathol 61:153-158,1956.