Institute of Pathology, City Hospital München-Schwabing, Kolner Platz 1, 8000 Munchen 40, West Germany
This paper is a continuum of a recent publication on fixation of the brain for plastination (Riepertinger, 1988). A technique is presented for removal, fixation and plastination of the central nervous system as a unit.
Brain; Spinal Chord; S10; Silicone; Biodur
Alfred Riepertinger Institute of Pathology, City Hospital München-Schwabing, Kolner Platz 1, 8000 Munchen 40, West Germany
![]()



This paper is a continuum of a recent publication on fixation of the brain for plastination (Riepertinger, 1988). A technique is presented for removal, fixation and plastination of the central nervous system as a unit.
Specimen Selection
Selection of a suitable corpse may assure a better specimen. Since osteoporotic bones are fragile and tend to fracture and result in unintentional crushing of the spinal cord, the age of the deceased should not be over 70 years. Similarly, corpses having carcinomas with skeletal metastasis are not recommended. Since the brain and the spinal cord autolyze quickly and are sensitive to crushing, the dissection should be done no later than 48 hours post-mortem.
Removal Technique
Within the scope of a post-mortem examination, the autopsy is begun by excision of the thoracic and abdominal viscera. Therefore, removal of the brain and spinal cord from a ventral approach is facilitated and preferred to a dorsal approach, since the corpse need not be turned over. During the test-series, all 15 brains with adherent spinal cords were removed via the ventral approach. Ten were removed in the fresh state and the other five after lumbar-injection with 5% formaldehyde solution.
First, the cranial cavity was opened circularly with a surgical swing saw. To remove the skull-cap with ease, the cap was loosened using a cross-chisel, Virchow's skull breaker. The medulla oblongata can easily be bruised or the region dilated during the craniotomy by repeated movement of the head.
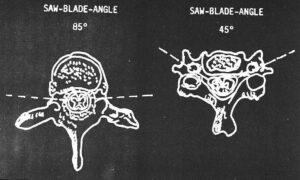
Figure 1. Diagram of the variation in the saw blade angle from the thoracolumbar region to the cervical region.
After the craniotomy was completed, the vertebral canal was sawed open with an oscillating saw fitted with a 20 mm wide blade. Medial disarticulation of the clavicles facilitated using the surgical swing-saw on the spinal column. Cutting the subclavicular muscle allowed the clavicles to be pulled laterally out of the way of the saw. To maintain the natural curvature of the spine, a stainless steel roll with rubber-naps was placed under the lumbar region of the cadaver and a rubber adjustable headrest under the neck. In the lumbar and thoracic regions, the blade of the oscillating saw was angled at 85 # from the sagittal plane and the pedicle was cut near the vertebral body (Fig. 1). Proceeding linearly, taking care not to damage the spinal nerves and ganglions, the pedicles were transected exposing the intervertebral foramina. It was important to control the depth of cut and thus prevent damage to the cord and roots. If the blade did not cut deep enough, the intervertebral foramina were not reached. However, the canal may be reached by sawing another bone fragment. In the cervical region, the angle of the saw blade was changed to 45# (Fig. 1). The cervical spine was unstable after the spinal canal was opened. Once the pedicles were transected, the ventral part of the spine (vertebral bodies and intervertebral discs) were removed. Virchow's skull breaker facilitated removal of the vertebral bodies and exposure of the vertebral canal containing the spinal cord.
Using surgical forceps and a scalpel, the spinal cord was removed, starting in the lumbar region and proceeding to the cervical region. By pulling each spinal nerve laterally as it emerged through the dura, the nerve was loosened and dissected free. Similarly, from the lumbar region to the cervical region, the dura mater was sectioned in the in situ fixed specimens. However, in fresh specimens, the dura was not opened at this time. As the dura traverses the foramen magnum, it was opened with a long, thin brain-knife around its circumference (until the cerebellum was seen) prior to removal of the brain.
To remove the brain, a pair of scissors was used to cut the dura mater along the circumference of the saw line and along the falx cerebri as it disappeared between the cerebral hemispheres. To expose all the nerves emerging from the brain, the brain was lifted up and held in hand and each nerve was cut. Using a long, thin brain-knife, the tentorium cerebelli was cut near the petrous portion of the temporal bone. When the brain was freed of all dural attachments and nerves, an assistant was utilized to take the brain cranially so that the spinal cord might be brought through the foramen magnum. To prevent damage to the fragile spinal cord, it must not be squeezed or stretched.
After the entire central nervous system was removed, the brain was placed into a previously prepared "Hedgehog mold" (brain-bowl) and fixed by vascular perfusion. The spinal cord was carefully placed on a 50 x 10 cm cardboard strip (dorsal surface toward the cardboard). The spinal cord must not touch the edge of the "Hedgehog mold" as this will result in pressure-points on the cord or it may be severed.
Fixation Via Lumbar-Injection in Situ with 5% Formalin Solution.
To perform a lumbar-injection into the subarachnoid space, the corpse was placed on its side with the hips and knees flexed and the spine flexed as much as possible. To remove cerebrospinal fluid, a 20 x 100 mm needle was placed through an intervertebral space of the second to fifth lumbar vertebra into the subarachnoid space. Approximately 45 to 65 ml of cerebrospinal fluid was drawn off and replaced with 80 to 100 ml of 5% formaldehyde solution. Fixation time was 20 to 24 hours, after which, removal was performed as described above.
Fixation Via Injection of the Brain with 100% Formalin into the Basilar Vessels.
After the brain was secured in the "Hedgehog mold", a polyethylene cannula (1.0 x 1.5 mm) was inserted into one of the vertebral arteries, reaching into the basilar artery. The other vertebral artery and both carotid arteries were ligated. Using light pressure, 150 ml of 100% formalin was injected. After perfusion, the tube was carefully pulled from the vertebral artery and the vessel was ligated.
Fixation Via Immersion of the Central Nervous System in a 5% Formaldehyde Solution.
After the brain had been perfused, the spinal cord's dorsum was placed on a cardboard strip, with the spinal nerves projecting laterally. The ends of the nerves were secured with pins or small gauge (0.45 x 13 mm) disposable hypodermic needles. If previously fixed via lumbar injection, the dura of the cord had been opened previously and was also pinned at this time. Immersion fixation, with 5% formaldehyde solution, was carried out in the "Munich-fixation-tube", a specially designed transparent perspex (plexiglas) 30 x 100 cm cylinder (Fig. 2). The upper rim of the cylinder was supplied with hooks on its outer surface to fasten the gauze which suspended the brain. The cylinder was filled with 60 liters of 5% formaldehyde, and a 80 x 80 cm gauze square was fastened to the hooks to support the brain. In the middle of the gauze an opening was cut so the cardboard- strip, with its attached spinal cord, could pass through the opening to be suspended in the fixative. To stretch the spinal cord especially in the medulla region, the cardboard-strip was weighted with 20 to 50 grams. The preparation was carefully immersed into the fixative allowing the gauze to suspend the brain. The brain should not be suspended by the basilar artery as the medulla and cord will flex dorsally and be distorted from their natural position. The gauze will not cause pressure marks since the brain was hardened by perfusion with 100% formalin. After 1 to 3 days of fixation, the preparation was removed from the fixative and the dura, if not previously opened, was opened ventrally and pinned to the cardboard-strip in a manner similar to the spinal nerves. The preparation was replaced into the fixative for another 4 to 6 days.
Dissection
After immersion-fixation of about one week, the preparation was removed from the fixative and rinsed in cold water for at least thirty minutes. Then the brain was placed into the "Hedgehog mold" and the cord prepared for dissection of the spinal nerves and ganglions. The brain and cord was kept moist during the dissection. The ganglions and nerves were carefully cleaned of all connective tissue. After dissection, the nerves and dura were again pinned to the cardboard.
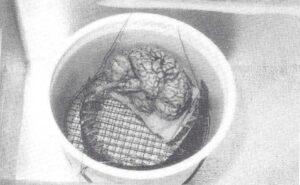
Figure 3. Photograph of the preparation coiled to the appropriate size for the dehydration vat and plastination chamber.
Dehydration
If the dehydration vat or plastination chamber was not long enough for the specimen to lie flat, the specimen was coiled to fit into the chamber (Fig. 3). Caution was used to prevent kinking the preparation. To hold the brain and cord in this position, they were carefully fastened with a string to a sturdy grid which would fit into the dehydration vat and/or the plastination chamber. Two strings were attached to the grid to allow immersion of the preparation into the cold acetone and removal from it. Freeze-substitution dehydration took thirty days, during which the acetone was changed twice: after 2 weeks and after 1 week. The preparation was left coiled until the end of forced impregnation.
Forced Impregnation
The preparation was placed into the polymer mix (Biodur polymer S 10, hardener S 3) on a Monday to allow the specimen and polymer to equilibrate and vacuum was started on Wednesday. Forced impregnation was in a deep freezer at minus 25#C and time of impregnation was seventeen days. After impregnation, to assure adequate time for equilibration, the preparation was left in the polymer over the weekend, at atmospheric pressure. Removal of the preparation on Monday morning, allowed a week for manicuring the preparation while it hardened.
Hardening
A container, long enough for the specimen to lay out flat (79 x 59 x 23 cm) was fitted with a grid which was covered with filter paper. Two containers, with 150 ml of Biodur gas hardener (S 6) and with plastic tubing leading to a pump, were placed on the grid along with a container of calcium chloride for absorption of moisture. Upon completion of forced impregnation, the specimen was removed from the vacuum kettle and the excess polymer wiped off. The spinal cord was removed from the cardboard-strip and again, the specimen was swabbed with a paper towel to remove excess polymer and was placed into the previously prepared gas-hardening chamber. By supporting the brain laterally with paper towels, the brain rested on its occipital lobes. During the first two days of hardening, the specimen was checked hourly and cleaned repeatedly of excess polymer using paper towels until the surface had a dull finish.
Usually after two days of gas cure and manicuring, the specimen was tacky and needed no more manicuring and hardening proceeded without the necessity of manicuring. When the surface was no longer tacky, the specimen was wrapped in polyethylene foil to allow the gas cure to penetrate the specimen. Every two days the foil was opened, the specimen was turned over, and any excess polymer was wiped off. When the specimen ceased to ooze, it was removed from the polyethylene wrapping. It took an additional eight to twelve weeks for the specimen to cure throughout its thickness (Fig. 4).
A ventral approach for removal of the central nervous system, as described above, is the preferred approach. The cord may be removed from the dorsum; however, the dorsal approach is not recommended as the spinal cord may be crushed when the corpse has to be turned over during the autopsy, and dissection of the spinal nerves and ganglions is difficult because of their position on the ventral aspect of the vertebral column.
To choose whether to remove the cord in the fixed or non-fixed state, the advantages and disadvantages of each state must be considered. The disadvantages associated with removal of the central nervous system in its fresh state, regardless of the approach, are the soft nature of the nervous tissue which renders it easily damaged and more sensitive to pressure marks especially in the region of the foramen magnum. Also, the dura mater may only be opened after one to three days of immersion fixation. These disadvantages can be prevented by subdural injection of fixative (5% formaldehyde solution) in the lumbar region. Subdural fixation strengthens the spinal cord especially in the region of the foramen magnum at the medulla oblongata. However after fixation, the dura is less flexible and may be damaged by the saw, and there is the risk of the cord's surface drying, unless the preparation is kept moistened. However, the advantages of removing the specimen in its fresh state include: the dura mater is unlikely to be damaged by the oscillating saw, during opening of the spinal canal, since it is pliable. Also, the cord does not dry out as easily.
For immersion-fixation of the central nervous system, the specially manufactured transparent cylindrical, "Munich-fixation-tube", is desirable, but not essential. For our first experiments, we used a PIastibrand flask with a height of 70 cm and a diameter of 46 cm. The disadvantages were the high volume of the 5% formaldehyde solution (110 liters) and the flask was not transparent to allow visual inspection of the preparation. Therefore, the Munich-fixation-tube was designed. Other type of cylinders may be used for immersion fixation.
After fixation, the preparation may be stored in another container filled with 5% formaldehyde solution. For long term storage in fixative, pinning of the nerves and hanging of the fixed specimen is not necessary. Other materials (mesh or nylon) may be used to suspend the brain.
Riepertinger A: Fixation of the Human Brain for Plastination: Special considerations. J Int Soc Plastination 2:8-12, 1988. https://doi.org/10.56507/XMBX2600