Anatomical Institute Karl-Franzens University Graz Graz, Austria
The S-10 procedure of plastination (von Hagens, 1986; von Hagens et al., 1987) is used for the preparation of half and whole brains as well as brain dissections. With the increasing difficulty in obtaining brains for neuroanatomy courses, the production of S-10 specimens has become important in teaching because these specimens can be kept in good condition for long periods of time before being replaced. S-10 plastination of 1.0-2.0 cm slices of brain tissues can also provide a useful teaching tool. The P-35 and P-40 methods of plastination are used for the production thin (4,6 or 8 mm), semitransparent slices of brain tissues. Discussed within this paper will be the methods, for the above mentioned procedures, that are used at the Anatomical Institute in Graz.
Brain; S10; P35; Brain Sections
Andreas H. Weiglein M.D. Anatomical Institute Karl-Franzens University Graz Graz, Austria
![]()



The S-10 procedure of plastination (von Hagens, 1986; von Hagens et al., 1987) is used for the preparation of half and whole brains as well as brain dissections. With the increasing difficulty in obtaining brains for neuroanatomy courses, the production of S-10 specimens has become important in teaching because these specimens can be kept in good condition for long periods of time before being replaced. S-10 plastination of 1.0-2.0 cm slices of brain tissues can also provide a useful teaching tool.
The P-35 and P-40 methods of plastination are used for the production thin (4,6 or 8 mm), semitransparent slices of brain tissues.
Discussed within this paper will be the methods, for the above mentioned procedures, that are used at the Anatomical Institute in Graz.
S-10 Technique Fixation:
Fresh brains are fixed in 10% Formaldehyde (14 days minimum), rinsed in cold tap water overnight and cooled to 5°C before use. Previously fixed brains (stored for long periods of time in fixative) are washed thoroughly in tap water and cooled to 5°C.
Dehydration:
Brains are submerged in at least two baths of 100% Acetone at -20°C (25L/brain). When the acetone con- centration remains at 99%, dehydration is complete (whole brains approx. 3 wks). Dehydration at -20°C is necessary in order to prevent shrinkage of the brains.
Immersion:
Brains are immersed in a mixture of S-10/S3 (100:1) for 1 week at -20°C. This step helps to prevent shrinkage in the specimens. Longer immersion at this stage will result in shorter impregnation time for the specimens.
Forced Impregnation:
Brains are placed under vacuum for three weeks at -20°C The vacuum is slowly increased until 1-2 mm of Hg is attained. Slow increase of vacuum helps to prevent shrinkage of the specimens.
Gas Curing:
After forced impregnation, the brains are removed from the polymer and excess polymer is drained from their surfaces. The specimens are then placed on a grid and exposed to S-6 (gas cure) vapors for 3 days at room temperature. The vaporization of the S-6 is enhanced by bubbling air through it using a small aquarium pump. This enables fast curing of the brains' surfaces. To assure complete curing of the polymer inside the specimens it is necessary to store them in air-tight plastic bags for an additional two (2) months.
Slicing:
Following curing, the brains can be sliced with a band saw. It is possible to slice very thin (1mm or less) slices from a thicker slice by using a meat slicer. After slicing, if the brain slices do not appear to be fully cured they can be returned to the curing chamber for another 24 hours.
Finishing:
After slicing, the brain slices can be sanded to a smooth finish on a belt sander. A constant supply of water to the surface of the specimens helps in this procedure.
P-35 Technique Fixation:
Fresh brains were fixed in 10% Formalin for 4-6 weeks. Specimens which have been fixed for longer periods of time or by other methods should be avoided for this procedure. Other fixation methods may alter the P-35 reaction.
Slicing:
After embedding in 20% gelatin (Barnett et al, 1980) brains were cut with a meat slicer into 4mm slices. To prevent degradation of the slices, after cutting and during subsequent handling, they where placed on a piece of wet filter paper before being transferred to stainless steel grids. The grids containing the slices were placed into a stainless steel basket for flushing.
Flushing:
The basket of slices was rinsed with cold tap water overnight and cooled to 5°C before proceeding.
Dehydration:
The basket of brain slices was placed in 100% Acetone at -20°C for 3 days.
Immersion#1:
The basket of brain slices was placed in a bath containing a mixture of P-35/A9 (100:2) for 24 hours at 5°C. Note: This bath may be the Immersion *2 bath from a previous run of sections.
Immersion #2:
The basket with the brain slices was placed in another bath of P-35/A9 (100-2) mixture for a further 24 hours at 5°C. Note: this bath may be the bath of resin used for forced impregnation during a previous run of specimens.
Forced Impregnation:
The basket of brain slices was transferred to a fresh mixture of P-35/A9 (100:2) and placed under vacuum for 24 hours at room temperature. The vacuum was increased until 10-15mm Hg was attained.
Casting:
The basket of slices was removed from the vacuum chamber, and individual slices were placed between two sheets of glass plates. Each sheet consisted of an outer piece of safety glass and an inner sheet of float glass, the latter sheet facing the specimen. A silicone gasket was placed between the outer edges of the sheets and then clamped in position using fold back clamps. The double glass chambers, containing the specimens, were filled with afresh mixture of P-35/A9 (100:2) mixture.
Light Curing:
After casting the double glass chambers were exposed to UVA light for a period of 3 hours. During this procedure it is necessary to cool the chambers either by ventilators or as described by Weiglein and Bahadori (1995).
Heat Curing:
Following light curing the double glass chambers were placed in a well ventilated oven at 45°C for 5 days.
Finishing:
When curing was completed the glass chambers were dismantled and the sections were trimmed on a band saw. After sawing the edges were smoothed using a belt sander.
| S-10 TECHNIQUE | P-35 TECHNIQUE | ||||
| Step | Time | Temperature | Step | Time | Temperature |
| FIXATION | 3 weeks | room temp. | FIXATION | 3 weeks | room temp. |
| Slicing | |||||
| Flushing & Cooling | 24 h | 5°C | Flushing & Cooling | 24 h | 5°C |
| DEHYDRATION | 3 weeks | -20°C | DEHYDRATION | 3 days | -20°C |
| Immersion | 1 week | -20°C | Immersion | 2x24 h | 5°C |
| FORCED IMPREGNATION | 3 weeks | -20°C | FORCED IMPREGNATION | 24 h | 5°C |
| Double Glass Chamber | |||||
| Gas-CURING | 3 days | room temp. | Light-CURING | 3h | room temp. |
| Post-CURING | 2 months | room temp. | Heat-CURING | 5 days | 45°C |
| Slicing | |||||
| Final Treatment | Final Treatment | ||||
P-40 Technique
This technique has advantages over the others. P-40 resin has a lower viscosity than P-35 resin (von Hagens, 1994). The advantages of using this technique are:
Experience with the use of this technique is minimal and will be discussed in further publications.
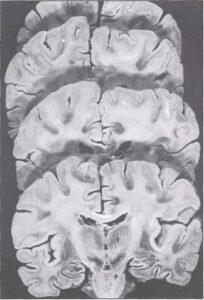
Fig. 2: S-10 plastinated brain sliced in coronal sections. The first slice shows the pyramidal tract .(P) running between the thalamus (T) and lentiform nucleus (L). Note distinction between gray and white matter.
Sliced S-10 plastinated brains provide a good teaching tool. They show good differentiation between white and gray matter in comparison to other types of fixed brains (Fig. 2). This differentiation may be enhanced by staining with such substances as Astra Blue or Aldehyde Fuchsin (Ulfig, 1990; Ulfig & Wuttke, 1990). Slice thickness can be varied from 0.5mm and up. Specimens are flexible and therefore less susceptible to damage.
P-35 plastinated brain slices provide an excellent tool for use in teaching and research. Semitransparent P-35 brain slices maybe observed under direct or transmitted light. They have excellent gray-white matter differentiation and provide gross microscopic detail of some nuclear areas (Fig. 3). P-35 sections provide better macroscopic detail than those sections stained by other methods such as Berlin Blue or Mulligan Staining.
Because of the variability in slice thickness, specimens can be used in a wide variety of ways such as:
8 mm slices may be employed to give the student a three dimensional concept of neurological
Barnett, R.I., Lyons,G.W., Driscoll, J.D., Forrest, WJ. 1980. Improved sectioning and Berlin Blue staining of whole human brains. Stain Technology 55/4:235-239.
https://doi.org/10.3109/10520298009067246
Ulfig, N. 1990. Staining of human fetal and adult brain slices combined with subsequent plastination. J Int Soc Plastination 4/1:33-38.
https://doi.org/10.56507/THKE9781
Ulfig, N., Wuttke, M. 1990. Plastination of stained sections of the human brain. AnatAnz 170:309-312.
von Hagens, G. 1986. Heidelberg Plastination Folder, 2nd Edition.
von Hagens, G. 1994. Plastination of brain slices according to the P-40 procedure. A step-by-step description, pp. 1-23.
https://doi.org/10.56507/OWYV2878
von Hagens, G., Tiedemann, K., Kriz, W. 1987. The current potential of plastination. Anat Embryol 175:411-421.
https://doi.org/10.1007/BF00309677
Weber, W. 1994 Sheet Plastination of brain slices. J Int Soc Plastination 8/1:23.
https://doi.org/10.56507/OWYV2878
Weiglein, A. 1993. Plastinated brain slices in the anatomical curriculum at Graz University. J Soc Plastination 7/1:3-7.
https://doi.org/10.56507/EHRX7749
Weiglein, A., Bahadori, K. 1996- Nerve Plexuses demonstrated by the P-35 Technique. J Soc Plastination 10 (1): 4-5.
https://doi.org/10.56507/UYCK9598