Biomedical Sciences, Cummings School of Veterinary Medicine Tufts University, North Grafton, MA
The plastination process produces large quantities of waste acetone contaminated with water and lipids dissolved in the solvent. Disposal creates environmental pollution and makes the plastination process more expensive. Recycling the acetone is the ideal solution. Like most plastination facilities, our plastination unit incorporates acetone recycling equipment. We describe how we have used the excess capacity of the recycling unit to help our hospital’s histopathology section to recycle their used alcohol and Pro-Par Clearant (a xylene substitute). As a result, they save some $7,000 a year (that were allocated towards purchasing new reagents), a part of which funds our plastination facility. The recycling plant is environmentally responsible and in line with the University’s ‘green’ initiatives. As of early 2014, we have recycled approximately 6624 L (1,750 gallons) of solvent for the hospital at $US3.3/L (12.50/gal), for a total income of almost $22,000.
acetone; alcohol; histopathology; recycling; solvent
Dr. Jack Hawkes, Biomedical Sciences Department, Cummings School of Veterinary Medicine at Tufts University, 200 Westboro Rd., North Grafton, Ma. 01536 Tel. +001 508 887 4674; Fax: +001 508 839 8787; E-mail: jack.hawkes@tufts.edu
![]()



The Cummings School of Veterinary Medicine’s plastination program was initiated in 2007, with the primary goal of reducing animal usage in our anatomy teaching program. The facility includes an acetone recycler to distill the acetone waste that is a byproduct of the plastination process. It was soon realized that the recycling unit could have wider use for recycling organic solvents generated by various laboratories on the campus. We describe here a mutually beneficial agreement with our school hospital’s histopathology section to recycle their used alcohol and Pro-Par clearant (a xylene substitute). The process involves picking up used solvent from the histopathology department, recycling it, and selling it back to them for half of the cost of new solvents (including what they would pay for shipping). They saved approximately $7,000 in 2013, and half these revenues (from resale to the histopathology lab) help fund the plastination program. In addition, the university saves the costs that would be involved in disposing of used solvents (which may equal 50% of the solvent’s purchase price(Taylor 2014)). Recycling solvents is environmentally friendly, as it reduces the consumption of petrochemicals, as well as the gasoline that would have been used to transport them (the xylene substitute is produced 1500 km from our campus). The combination of environmental sensitivity and fiscal responsibility fostered by the recycling program has also won recognition from Tufts University as a ‘green’ initiative. As of early 2014, we have recycled approximately 6624 L (1750 gallons) of solvent for the hospital at $3.3/L (12.50/gal), for a total income of almost $22,000. We typically recycle between twenty and twenty-five gallons of histopathology solvents a month, in addition to processing roughly fifty-five gallons of acetone for our lab needs. In this paper, we describe the recycling process involved in distilling histopathology solvents on the campus and the monetary benefit.
On the other hand, such a program will not work without a reasonable commitment on the part of both parties toward making the program work. Problems like contamination will inevitably crop up, and it’s important to have procedures in place to deal with them. The success of our program is due in large part to the institution’s support of recycling and innovation.
There are important physical limitations to recycling. Most importantly, alcohol is an azeotrope, a liquid whose concentration can only be changed to a certain point by distillation, because the liquid and the vapor it produces have the same composition. In the case of ethanol, for example, pure water of course boils at 100°C; pure ethanol boils at 78.3°C; but a mixture of 95.63% ethanol and 4.37% water (by weight) boils at a lower temperature,78.2°C, so by conventional distillation, ethanol can only be recycled to about 95% purity. Higher purity (100%) alcohol is produced on an industrial scale either through addition of an entraining agent like benzene, or by removing the water with a desiccant (molecular sieves).
Also, alcohol is sensitive to contamination by xylene or xylene substitutes. Alcohol which contains even small amounts of xylene or xylene substitutes cannot be completely purified by recycling, because these compounds cannot be completely removed. The mixture of water, alcohol and xylene, for example, forms a complicated azeotropic mixture with each of the three isomers (ortho, meta and para) of xylene (Fele et al., 2000)
The Histopathology Process
In order to understand histopathology solvent recycling, it may be helpful to review the process of tissue preparation in the histopathology lab (Fig. 1).
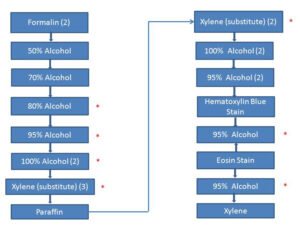
Figure 1. Overview of tissue embedding and staining. Fixed tissue goes into two changes of formalin (upper left), is dehydrated through six changes of alcohol, and embedded in paraffin (bottom left). The resulting block is sliced, slices are mounted on slides, and slices are cleared of paraffin (upper right) and stained (lower right). Solvent baths marked with an asterisk are recycled.
The first stages in tissue preparation are similar to the process of plastination: tissue is first fixed and then dehydrated in alcohol prior to embedding in paraffin. The fixed tissue sample goes through two changes of fresh formalin, then increasing concentrations of alcohol through 100%, finally through two changes of xylene substitute before being embedded in paraffin. The resulting block is sectioned and mounted on a slide. At this point, the process is reversed (xylene replaced with alcohol) so that the tissue section may be stained with water-based dyes. The slide is then cover-slipped.
Key points: There are two primary solvents used for histopathology: alcohol and xylene (or xylene substitutes). Alcohol is miscible in water and removes water from the tissue specimen. Xylene is miscible in both alcohol and paraffin, and thus aids paraffin to infiltrate the tissue. Xylene is not miscible in water. When water is added to an alcohol-xylene solution (even one containing a minute quantity of xylene), the xylene first causes optical distortion (cloudiness), then comes out of solution. If xylene remains in the final stages of the slide-making process it can cause stain spotting on the slide, ruining it. The consequences of solvent contamination are serious, and precautions against it must be taken.
Recycling Regulations
An initial step involved in recycling solvents is to make sure that the process meets the requirements of regulatory bodies. Anyone contemplating recycling alcohol, in particular ethanol, should verify (and document) the legal requirements of their activities. In the United States the Bureau of Alcohol, Tobacco and Firearms, a division of the Department of Justice, is the appropriate regulatory body. Denatured alcohol is ethanol to which methanol or isopropanol (or both) has been added to make it unfit for human consumption. Since these alcohols cannot be removed from ethanol, distillation should not pose a legal problem. Ethanol is alcohol which is fit for human consumption, and which requires obtaining a tax stamp for purchase. The ATF agency informed us that distillation of dirty ethanol for which a tax stamp had been purchased would be allowable under their rules, since the purpose of distillation would be simply to clean it and to return it to its as-purchased state. It goes without saying that liquids that have been distilled in a unit that has been used for plastination solvents are unfit for human consumption. However, anyone contemplating ethanol recycling would be well-advised to get written clarification of the law from the appropriate regulatory body before proceeding. Companies that engage in commercial solvent recycling are heavily regulated by a variety of agencies. For this reason we do not recycle any solvents for clients outside the university system.
Equipment
A Procycler A (Fig. 2) (B/R Instruments, Easton, MD, USA), was purchased in 2007 for $13,400 and has been operating daily for seven years, with only a few minor problems (which B/R Instruments have been extremely helpful in resolving).
When the recycler was purchased, we did not foresee recycling xylene substitutes, so we purchased a single-column recycler, at a saving of some $5,000. Generally, xylene/alcohol recyclers sold have two boilers and two columns, one for each solvent, while alcohol (or acetone) recyclers have only one column. A single column recycler may be used for xylene. However, since xylene’s boiling point is higher than that of water, recycling xylene is a two-stage process with our single column recycler. First, water and alcohol (which have lower boiling points) are distilled off, and the product/distillate is discarded. Next xylene is distilled (primarily to separate xylene from paraffin dissolved in it), and the product/xylene is saved. Since alcohol is very susceptible to contamination by xylene (and xylene substitutes), for the first two years of operation two batches of acetone were run between Pro-Par and alcohol to ensure that there was no residual Pro-Par in the recycler. A two-column distillation unit, which has a separate boiler and column for alcohol and xylene, prevents this problem.
Specific Gravity
Our primary tool for analysis is the hydrometer (for alcohol, one calibrated to read in proof). The specific gravity of every batch of histopath solvents we run (and a sampling of the acetone batches we run, as well) is recorded. Ideally, these tests would be run at a standardized temperature (normally 20° C), but because it is quite cumbersome to run these tests at standardized temperatures, we have either found tables or charts of the solvents we use at various concentrations and temperatures, or have constructed charts of our previous results (to use as a rough guide). Results are thus obtained considerably more quickly, and possibly more accurately as well, since results don’t depend on attempting to record a solvent’s specific gravity at the moment it warms up to the temperature for a hydrometer is calibrated.
The U.S. National Bureau of Standards has published a very complete table, entitled “The True Percents of Proof Spirit for any Indicationof the Hydrometer at Temperatures Between 0° and 100° F.” (available at http://www.ttb.gov/foia/Gauging_Manual_Tables/Table_1.pdf), which allows compensation for any temperature between 0° F and 100° F at any proof from 1 to 200 (proof, of course, is simply double the percentage of ethanol). It should be noted that the alcohol we distill, as previously noted, has been denatured. It consists of approximately 85% ethanol (S.G. 0.789), approximately 5% methanol (S.G. 0.792), approximately 5% isopropanol (S.G. 0.786), and at least 5% water. For simplicity’s sake, and because we are primarily concerned with consistency from batch to batch, we assume our alcohol to be composed entirely of ethanol. Note that if ethanol is denatured with equal volumes of methanol and isopropanol the resulting specific gravity is the same as for pure ethanol, since isopropanol’s S.G. is 0.003 less than that of ethanol, while methanol’s is 0.003 greater.
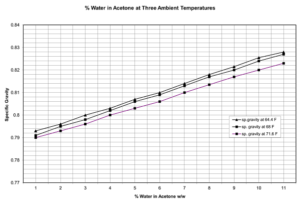
Figure 3. Chart of water percent in acetone by specific gravity at three temperatures, constructed from data published by the University of Stirling, Scotland (http://www.thin.stir.ac.uk/tag/drying/).
For acetone, we use a chart (Fig. 3), constructed from data published by the University of Stirling (http://www.thin.stir.ac.uk/tag/drying/), which gives the specific gravities for water contents between 0–11% at three temperatures: 71.6°F, 68°F and 64.4°F. This range covers the temperatures normally found in our acetone; for temperatures in between the data points we extrapolate: for example, a hydrometer reading of 0.800 indicates acetone that is 97% pure at 64.4° F, 96.5% at 68°F, and 96% at 71.6° F. The acetone from our recycler averages 97% purity. For Pro-Par, we have constructed a table of the specific gravities we have recorded at various temperatures in the past; this allows us to identify any batch which falls outside the norms of what we’ve seen in the past.
Contamination
We carefully check for xylene (or xylene substitute) contamination of every batch of alcohol we recycle. Xylene is miscible in alcohol, but not miscible in water. If a few drops of contaminated alcohol are floated on water, the xylene will come out of solution in the alcohol, and be seen as an oily sheen on the water (this is how we used to test for contamination). At concentrations lower than those which will force the xylene out of solution, xylene has the effect of disturbing the optical qualities of the alcohol/water solution, making it cloudy; this is a variation of the histological technique for checking or determining the purity of alcohol using xylene (Humason, 1962; Hall, 2001). We have found this to be the more sensitive visual test.
Pro-Par
Finally, there are a couple of tricks we have picked up in recycling the xylene substitute used by our histopathology lab. Manufactured by Anatech, Ltd. (Battle Creek, MI, USA; http://www.anatechltdusa.com/), Pro-Par is a xylene substitute (technically, it is a propylene glycol ether) marketed for, among other things, its ability to be recycled. The distillate of Pro-Par has a very strong and objectionable fishy odor; for this reason, the distillate is filtered through activated charcoal, which removes the odor. Pro-Par attacks many plastics, as well as cured silicone adhesive. For this reason we use refillable filter cartridges (Catalog No. 9007T53, McMaster-Carr, NJ, USA), and dispose of the cartridge after each use. We change filters approximately every six months.
Quality Control
Because some histopath samples (i.e. biopsies) are irreplaceable, it is important to work closely with the clients of recycled solvent, and to discuss how to handle problems with solvent quality before they happen. Quality control of recycled solvents is critical. Even small amounts of Pro-Par in recycled alcohol, for example, can cause spotting on slides when they are stained, ruining the biopsy sample.
Last year Pro-Par contamination of our alcohol was a problem. Pro-Par is soluble in fat. Investigation revealed that the acetone being distilled between Pro-Par and alcohol (to clean the unit) was very high in fat content. The fat residue which remained in the boiler absorbed Pro-Par which then contaminated the alcohol during its recycling period. As a result of this incident, we purchased a separate boiler dedicated to alcohol recycling (at a cost of $2,250), although this step is probably only financially justifiable for larger programs. We also instituted rigorous quality control measures, which we now recommend to anyone contemplating recycling histopathology solvents; more limited quality control measures will probably benefit those who only recycle acetone, by monitoring quality and banking samples in case of future problems.
It is important to maintain a daily record of the recycling activity. We record the history of each batch of solvents we recycle. Quality analysis of the recycled solvent is also important. We record the specific gravity of each batch of solvent we run, and bank samples of all the solvents we recycle. Every container of recycled solvent is marked with its batch number, so if there turns out to be a problem with it, the banked sample can be qualitatively analyzed to pinpoint the problem.
We have found that making use of our recycler’s unused capacity for recycling solvents for the histopathology department in our school’s hospital has several benefits. It converts unused capacity to income, and helps meet the school’s goals of environmental responsibility. Recycling has taught us a great deal about maximizing the quality of recycling for all our solvents. Finally, in these days of cost-consciousness, the evidence of fiscal responsibility provided by this business arrangement helps ensure that we are able to continue our plastination program.
Fele, L, Štemberger, N, Grilc, V. 2000: Separation of water + ethanol + (o-, m-, p-) xylene systems. J Chem Eng Data 45: 784-791.
https://doi.org/10.1021/je990321u
Hall, J, 2001: A simple, rapid method for measuring the percentage of water in alcohols used for dehydrating tissues. Biotech & Histochem 76: 41- 42
https://doi.org/10.1080/bih.76.1.41.42
Humason, G, 1962: Animal Tissue Techniques. San Francisco: W.H. Freeman, p. 32.
https://doi.org/10.5962/bhl.title.5890
Taylor, J. 2014: Cost Benefit Analysis furnished by James Taylor, B/R Instruments, personal communication 2/21/14.