1- Department of Biomedical Sciences and Pathobiology, Virginia Maryland Regional College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061- 0442, USA.
2- College of Pharmacy and Health Sciences, University of Louisiana at Monroe, Monroe, LA 71209, USA
The technique of plastination requires the handling of chemical agents not normally encountered by anatomic preparators. Material safety data sheets that come with these chemicals provide limited information regarding potential toxic effects following skin, inhalational or other exposures to these compounds. Biodur™ S10-type silicones that are commonly used in plastination are polydialkyl siloxanes and are widely used in industry. A review of chemical safety sheets from industrial sources that produce such siloxanes suggests limited toxic effects associated with handling these agents. In contrast, a variety of adverse chemical effects including skin hypersensitivity-type responses have been associated with use of Biodur™ S6 and Biodur™ S3-like compounds. Epoxy resins commonly used by plastinators are well-recognized skin, eye and mucous membrane irritants and have been associated with allergic skin responses. Such toxic effects associated with the more common plastination chemicals are discussed in this paper.
chemical safety; plastination; siloxane
S. D. HOLLADAY: Telephone: 540-231-3372; Fax: 540-231-6033; E-mail: holladay@vt.edu
![]()



Health concerns associated with the plastination procedure are most commonly divided into two areas: 1. possible toxic effects of the chemicals used by plastinators and 2. risk associated with infectious agents contained in the tissues handled by plastinators. Regarding chemical toxicity, the proprietary nature of plastination chemicals has resulted in limitations on available data. Specifically, the precise chemical identity of plastination components (e.g., S10 silicone; gas cure, S3 catalyst/chain extender) is not public information, thus restricting literature searches for relevant information on undesirable effects from exposure to these compounds. Material Safety Data Sheets (MSDSs) have been available but are limited at best in terms of the information provided. Nonetheless, sufficient information is available for many of these chemicals to provide plastinators with expanded information on potential chemical toxicity. Silicone monomers and associated catalysts and hardeners and epoxy resins are common agents used for tissue impregnation during plastination. These chemicals are the focus of this paper.
Background: Chemistry of Silicone Plastination
Some understanding of silicone chemistry as it relates to plastination is helpful before discussing potential adverse effects of these agents. Biodur™ S10 is the most commonly used plastination chemical for tissue impregnation. This silicone product is a polyalkyl siloxane (Biodur™ S10 MSDS, Nov. 1998). Many closely related siloxanes are commercially available and widely used with S10-like compounds typically having methyl groups as the alkyl substitutions. However, the nature of the S3 hardener (a tin compound: Biodur™ S3 MSDS, Jan. 1999) suggests the reactive end groups on the S10 siloxane monomer are hydroxy 1 groups. Together these observations suggest the polyalkyl siloxane making up Biodur S10 is likely to be a hydroxyl-terminated polydimethyl siloxane or very similar silicone monomer. The chemical structure of this molecule is shown below (Fig. 1).
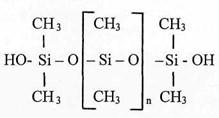
Figure 1. Structure of hydroxyl-terminated polydimethyl siloxane. [Si - O] represents a basic silicone molecule.
Toxicity of Polydimethyl Siloxane, Catalyst, and Cross-linker:
The Biodur™ S10 MSDS provides limited toxicity data for this compound. The following first aid measures are indicated:
After eye contact: Rinse with plenty of water. A doctor should be contacted immediately.
After skin contact: Remove product mechanically.
After swallowing: Seek medical advice.
We were surprised to find that industrial MSDSs for polydimethyl siloxanes similarly lacked specific information regarding chemical toxicity. A representative sheet obtained from United Chemical Technologies, Inc. (Bristol, PA, USA), which produces siloxanes including hydroxyl-terminated polydimethyl siloxanes, simply indicated, "No toxicity information available" for this silicone product. However they recommended that adequate ventilation, impervious gloves and safety glasses be used. Suggested first aid procedures were nearly identical to measures listed on the Biodur™ S10 MSDS and included:
After eye contact: Rinse with plenty of water. A doctor should be contacted immediately.
After skin contact: Remove product mechanically. After swallowing: Seek medical advice.
Given the large-scale industrial use of polydimethyl siloxanes for the past 20 or more years, these minimal cautions on the MSDSs suggest that limited toxicity has been observed in individuals working with polydimethyl siloxanes. The possibility of topical hypersensitivity (allergic) responses in siloxane- exposed individuals, such as may occur in individuals who become sensitized to formaldehyde, has been a concern expressed by some plastinators. This potential toxicity was not mentioned on MSDSs we obtained with the exception of one sheet from Imperial, Inc. (Green Bay, WI, USA) for a room temperature vulcanizing (RTV) silicone product that was 70-80% hydroxyl-terminated polydimethyl siloxane. This particular product contained low levels of other organosilanes, as well as carbon black. The MSDS indicated that the product may enhance allergic conditions in certain people. We contacted company representatives regarding this possible effect and requested additional information about the specific component of their product (siloxane or carbon black) that may lead to allergic responses. Unfortunately, Imperial, Inc. declined our request for more detailed information about the potential allergenicity of their product. In that carbon black is a suspected allergen (Anderson et al., 1992), the Imperial, Inc. MSDS reference to allergic responses may be a reflection of this constituent of this particular siloxane product.
In a response to an e-mail inquiry about hyper- sensitivity responses following skin contact with siloxanes, a chemist and technical manager at United Chemical Technologies, Inc. indicated, "to our knowledge none of these materials show allergic responses in animals or humans." It is interesting to note that Silly Putty® (Dow Corning, USA), a putty- like toy sold in the USA for the past 30 years, contains 65% hydroxyl-terminated dimethylsiloxane polymers (ref: http://www.thebuzz.net/ingredie.htm). Large numbers of children and others have handled this product without reports of skin allergic responses or other adverse health effects. It should also be pointed out that considerable human and animal data exist regarding possible adverse health effects from internal exposure to polydimethyl siloxane, in the form of silicone breast implants. The postulated relationship between breast implants and increased risk of autoimmune disease has generated intense medical and legal interest during the past 10 years. It now appears probable that these implants do not increase the risk of aberrant immune responses in humans (Janowsky et al., 2000). Thus, the available data suggest limited adverse immune or other toxic responses from exposure to silicone monomers similar to those used in plastination. Biodur™ gas cure S6 is described as an alkyl-silicate (S6 MSDS, Feb. 1999). Under the heading "Regulatory Information," the MSDS indicates S6 is harmful by inhalation and irritating to the eyes and respiratory system. Under the "Information on Toxicity" section, S6 is described as slightly irritating to eyes and moderately irritating to skin. Although the precise chemical nature of gas cure S6 is not provided, ethyl silicate (Fig. 2) is a physically similar, highly effective, common and inexpensive chemical used to cross-link polyalkylsiloxanes.
Figure 2. Structure of ethyl silicate. Si - refers to the silicone nucleus, which is covalently linked to four ethoxy groups.
Other related cross-linkers (e.g., ethyl polysilicate) may be used with equal success and show similar toxicity to ethyl silicate. The MSDSs provided by the U.S. Occupational Safety and Health Administration (OSHA; http://www.osha-slc.gOv//SLTC/healthguidelin es/ethylsilicate/recognition. html#healthhazard) summa- rizes potential toxic effects in humans exposed to ethyl silicate as follows:
Ethyl silicate is an eye, mucous membrane and respiratory irritant in humans. By analogy with effects seen in animals, it may also cause liver and kidney damage, central nervous system depression and anemia. At concentrations of 3,000 ppm, ethyl silicate causes extreme and intolerable irritation of the eyes and mucous membranes; at 1,200 ppm, it produces tearing of the eyes; at 700 ppm, it causes mild stinging of the eyes and nose; and at 250 ppm, it produces slight irritation of the eyes and nose (Hathaway et al., 1991).
The effects described by OSHA at the lower end of these exposures (i.e., 250 - 700 ppm) may be experienced by individuals who use S6 and/or ethyl silicate with inadequate ventilation. These effects are clearly avoidable with proper use of hoods. It should be remembered that cross-linkers of this type are highly reactive to available hydroxyl groups (i.e., H-OH) such as those in moisture on the surface of the eyes, mucus membranes and respiratory tract. Therefore, nearly all water must be removed from tissues before impregnated S10 silicone can be hardened. If not, the cross-linker will readily bind available water molecules on one or more of its four cross-linking arms instead of binding silicone monomer. This inhibits the silicone hardening reaction. In like manner, inhaled cross-linker will react with water in the lungs, and may thereby contribute to pulmonary silicosis. Ethyl silicate can cause severe damage to the eyes should the liquid form (as compared to gas vapors) be inadvertently splashed into the eyes. The MSDS provided by United Chemical Technologies, Inc. for ethyl polysilicate cross-linker indicates, for such eye contact, eyes should be flushed with clean water for at least 15 minutes and medical attention should be sought immediately.
Acute exposure to high levels of ethyl silicate may cause respiratory difficulty, tremor, fatigue, narcosis, nausea and vomiting (Sittig, 1991). Such exposures should be unlikely in plastinators who practice reasonable levels of caution while handling S6 cross- linker. Prolonged or repeated exposure of the skin to ethyl silicate has been reported to cause dermatitis (Genium, 1989). In summary, the available data suggest that plastinators should take care to limit their exposure to S6 vapors. These vapors may irritate the eyes and potentially deposit silicone into the lungs. Contact with the S6 liquid on skin or, especially, eyes should also clearly be avoided.
As we mention above, Biodur™ hardener S3 is described as a tin compound, specifically a dialkyl tin ester. A compound that has these characteristics and is used to join polyalkyl siloxane monomer chains end-to- end is dibutyltin dilaurate (Fig. 3).
Under the heading "Regulatory Information", the MSDS indicates S3 may be harmful if inhaled or swallowed and is irritating to eyes. It is also indicated that S3 can be handled without risk to health if used properly according to specification and if usual precautions of industrial hygiene are observed. The precise chemical nature of S3 is again unknown. However, relatively high-level exposure to related tin catalysts has been reported to produce hematopoietic (Subramoniam et al., 1994) and central nervous system (Alam et al., 1988) effects in laboratory rodents. The MSDS provided by OSHA (http://www.osha- slc.gov/ChemSamp_data/CH_271900.html) for dibutyl- tin dilaurate indicates health affects that include irritation to the eyes, nose, throat and skin. Acute exposure to high doses of dibutyltin dilaurate (which again should not be a problem in a typical plastination setting) may cause headaches, vertigo, eye irritation, psycho-neurological disturbance, sore throat, coughing, abdominal pain, vomiting, urine retention, paresis, focal anesthesia, skin burns and pruritis. Thus, available data for S3-like tin catalysts suggest that eye and respiratory exposure to vapors should be avoided as should skin contact with the liquid agent. Similar to S6, this suggests that these agents should be used under a hood while wearing protective gloves and eye-cover.
Various epoxy resins such as Biodur™ E12 are used for preparing thin transparent body or organ slices. The E12 resin is hardened by addition of polyamine compounds (Biodur™ Hardener El MSDS, Jan. 1999). The nature of the amine mixture that is used to harden El2 is unknown and difficult to predict, however it should be considered that amines can be highly toxic compounds. The MSDS cautions that El is an irritant and poisonous if swallowed, inhaled or absorbed by skin contact. The following are recommended:
Eye contact: Rinse immediately with plenty of water
and seek medical advice. Skin contact: Wash immediately with plenty of water
and soap. In case of accident or sickness: Immediately contact a doctor.
Prior to hardening of E12 epoxy resin using El polyamines, the skin, eyes or respiratory tract of the preparator may be exposed to the epoxy resin or to resin vapors. The Biodur™ E12 MSDS (May, 1998) again provides limited toxicity data for this plastination compound. The following are listed as first aid measures after contact by various routes:
After inhalation: Supply fresh air and, for safety reasons, call a doctor.
After skin contact: Wash immediately with water and soap and rinse thoroughly. After eye contact: Immediately rinse opened eye for several minutes under running water. Consult a doctor immediately.
After swallowing: In case of persistent symptoms, consult a doctor.
The acute toxicity information listed is based primarily on the acute toxicity of the bisphenol A (epichlorhydrine) component which has an oral LD50 of > 10,000 mg/kg (rat) and a dermal LD50 of >2,000 mg/kg (rat). However, bisphenol A is an estrogenic compound, and as such, potential endocrine disrupting effects have recently become of concern (Ashby and Odum, 1998). Thus, limiting skin exposure to Biodur epoxy should be recommended.
Regarding personal protection, MSDS recommends the following for individuals working with E12:
Respiratory protection: Not necessary if room is well- ventilated.
Hand protection: Plastic gloves.
Eye protection: Tightly sealed safety glasses.
Body protection: Protective work clothing. Epoxy resins are well-recognized skin, eye and mucous membrane irritants. We obtained MSDSs for epoxy resins from a variety of industrial sources, and all indicated that the resins might cause skin and eye irritation. In addition to primary irritation, allergic sensitization may also occur after skin contact with epoxy resins. This potential for an allergic skin reaction was less commonly mentioned on MSDSs we surveyed. However, there are several literature references documenting both skin irritation and allergic dermatitis associated with epoxy resins. Wyoto et al. (1976) found that workers who came into contact with epoxy resins and their hardeners in the workplace had increased positive skin tests and skin lesions with time of employment. Kanerva et al. (1997) described allergic contact dermatitis as well as allergic rhinitis and an immediate contact skin reaction from a component of epoxy resins, methylhexahydrophthalic anhydride. Jolandi et al. (1990) found that allergic contact dermatitis was induced by several components of epoxy resins including amine hardeners, epoxy acrylates and the diglycidyl ether of bisphenol A. Beyond allergic dermatitis, numerous reports have described occupational asthma resulting from inhalation of epoxy resin vapors (Nielsen et al., 1989; Liss et al., 1993; Grammer, et al., 1994; Yokota et al., 1997; Bernstein, 1997). For the latter reports, various anhydride components of the epoxy compounds were generally regarded to be the allergic components. These collective reports suggest that it is prudent to avoid inhalation of vapors while working with epoxy resins. We believe these collective data are sufficient to recommend that Biodur™ epoxy be used under a hood rather than simply in a well-ventilated room.
Alam MS, Husain R, Srivastava SP, Seth PK. 1988: Influence of di-butyltin dilaurate on brain neurotransmitter systems and behavior in rats. Arch Toxicol 61:373-377.
https://doi.org/10.1007/BF00334618
Anderson KR, Avol EL, Edwards SA, Shamoo DA, Peng RC, Linn WE, Hackney JD. 1992: Controlled exposures of volunteers to respirable carbon and sulfuric acid aerosols. J Air Waste Manage Assoc 42:770-776.
https://doi.org/10.1080/10473289.1992.10467028
Ashby J, Odum J. 1998: The importance of protocol design and data reporting to research on endocrine disruption. Environ Health Perspect 106(7)A:315-317.
https://doi.org/10.2307/3434050
Bernstein DI. 1997: Allergic reactions to workplace allergens. JAMA 278(22): 1907-1913.
https://doi.org/10.1001/jama.278.22.1907
Genium. Material Safety Data Sheet No. 446. 1989: Schenectady, NY: Genium Publishing Corporation.
Grammer LC, Shaughnessy MA, Lowenthal M, Yarnold PR. 1994: Risk factors for immunologically mediated respiratory disease from hexahydrophthalic anhydride. J Occup Med 36:642-646.
Hathaway GJ, Proctor NH, Hughes JP, Fischman ML. 1991: Proctor and Hughes'chemical hazards of the workplace, 3rd ed. New York, NY: Van Nostrand Reinhold.
Janowsky EC, Kupper LL, Hulka BS. 2000: Metanalysis of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med 342:781-790.
https://doi.org/10.1056/NEJM200003163421105
Jolandi R, Kanerva L, Estlander T, Tarvainen K, Keskinen H, Henriks-Eckerman ML. 1990: Occupational dermatoses from epoxy resin compounds. Contact Dermatitis 23:172-183.
https://doi.org/10.1111/j.1600-0536.1990.tb04779.x
Kanerva L, Hyry H, Jolanki R, Hytonen M, Estlander T. 1997: Delayed and immediate allergy caused by methylhexahydrophthalic anhydride. Contact Dermatitis (CODEDG)36:34-38.
https://doi.org/10.1111/j.1600-0536.1997.tb00919.x
Liss GM, Bernstein D, Genesove L, Roos JO, Lim J. 1993: Assessment of risk factors for IgE-mediated sensitization to tetrachlorophthalic anhydride. J Allergy Clin Immunol 92:237-247.
https://doi.org/10.1016/0091-6749(93)90167-E
Nielsen J, Welinder H, Skerfving S. 1989: Allergic airway disease caused by methyl tetrahydrophthalic anhydride in epoxy resin. Scand J Work Environ Health 15:154-155.
https://doi.org/10.5271/sjweh.1869
Sittig M. 1991: Handbook of toxic and hazardous chemical, 3rd ed. Park Ridge, NJ: Noyes Publications.
Subramoniam A, Khandelwal S, Dwivedi PD, Khanna S, Shanker R. 1994: Dibutyltin dilaurate induced thymic atrophy and modulation of phosphoinositide pathway of cell signaling in thymocytes of rats. Immunopharmacol Immunotoxicol 16:645-677.
https://doi.org/10.3109/08923979409019744
Woyto A, Blizanowska A, Wsik F, Woyto J, Sward J, Baranowski H, Szacki R. 1976: Evaluation of the influence of epoxide resins and their hardeners on the female body. I. Skin tests. Arch Immunol Ther Exp (Warsz) 24:911-918.
Yokota K, Johyama Y, Yamaguchi K, Fujiki Y, Takeshita T, Morimoto K. 1997: Risk factors for sensitisation to methyltetrahydrophthalic anhydride. Occup Environ Med 1997 54:667-670.
https://doi.org/10.1136/oem.54.9.667