Department of Animal Science The University of Tennessee, College of Veterinary Medicine P.O. Box 1071, Knoxville, TN 37901-1071
Preparation of beautiful and anatomically correct whole lung preparations is difficult using the standard S10 forced vacuum impregnation technique. Lungs which have been saturated with solvent and later with polymer are difficult to cure, such that their proper anatomical shape is retained. If lungs in the fresh state are dried in their proper anatomical configuration, they can be plastinated using a modified forced air impregnation method. Instead of submerging the lungs in polymer and using vacuum for impregnation, a polymer-xylene mixture is poured into the tracheo-bronchial tree and air is used to impregnate the lung tissue thus leaving the alveoli empty.
Lungs;silicone;S10
Robert W. Henry Department of Animal Science The University of Tennessee, College of Veterinary Medicine P.O. Box 1071, Knoxville, TN 37901-1071
![]()



Preparation of beautiful and anatomically correct whole lung preparations is difficult using the standard S10 forced vacuum impregnation technique. Lungs which have been saturated with solvent and later with polymer are difficult to cure, such that their proper anatomical shape is retained. If lungs in the fresh state are dried in their proper anatomical configuration, they can be plastinated using a modified forced air impregnation method. Instead of submerging the lungs in polymer and using vacuum for impregnation, a polymer-xylene mixture is poured into the tracheo-bronchial tree and air is used to impregnate the lung tissue thus leaving the alveoli empty.
Fresh lungs were collected from necropsy and from a slaughter house. The heart, mediastinal tissue and fat were carefully separated from the lungs and tracheobronchial tree. A cannula or tubing of the appropriate diameter was ligated in the trachea. A water source was attached to the cannula and the lungs gently inflated to near capacity. After filling, the water source was removed and the water and blood allowed to flow out of the trachea. This flushing procedure was repeated 8 to 1O times, which cleared much of the blood from the lungs. The lungs were covered with moist toweling to prevent drying. Finally, the lungs were connected to the water source for over night flushing. Flow was adjusted so that the lungs remained inflated as blood/water mixture flowed out of the pulmonary vessels and parenchyma. The next morning, provided the blood had been flushed sufficiently, the water source was removed and the water allowed to drain from the lungs via the trachea. After the majority of the water was drained, the lungs were placed ontheir dorsal (posterior) surface and the cannula was attached to the laboratory air compressor. The air flow was increased until the lungs were inflated and remained inflated to near capacity. This flow was maintained until the lungs were thoroughly dried. To assure that the lungs remained
inflated, air-flow was monitored at one-half hour intervals the first day.
Larger specimens (pig, cow and horse) were fixed by injecting 1-5 ml of 100% formaldehyde via a syringe and needle through the cannula wall, thus allowing the air to carry the formaldehyde throughout the lung. Smaller specimens (feline and canine) often were not fixed. To prevent the lungs from sticking to the counter as they dried, as soon as specimens could be picked up and retain their shape, they were suspended by the trachea. Air was allowed to flow through the lungs continually until the specimen was completely dry. Drying time was dependent on specimen size (cats:1-2 days, dogs: 2-3 days, pigs and sheep: 3-6 days, cows and horses: 5-15 days). The lungs felt very light when completely dry.
After drying was complete, a polymer-xylene mixture, 5-1O times the weight of the dried lungs, was poured into the trachea. Six variations of polymer-xylene mixture were utilized in canine lungs of similar size. The percentage of xylene ranged from 15 to 40% at 5% increments. Mixtures of 30 and 40% were used In two each of feline, porcine, bovine, and ovine lungs. The lung was positioned so that as one-half of the polymer was poured into the trachea it flowed into the bronchi of the right side. The lung was then turned so that the other one-half of the polymer, when poured, flowed into the bronchiof the left side.
Immediately, after the polymer mixture was poured into the trachea, the air source was secured to the cannula in the trachea and air flow was started. Air flow velocity was not measured. However, nearly full flow from the laboratory air supply was used. During the first fifteen minutes of air impregnation, the lung was turned in many directions to allow maximal availability of polymer to all areas of the lungs. Within a few minutes, small beads of polymer mix were observed on the surface of the lung. Maximal air flow was continued for four to ten days and the lungs were inverted daily to assure maximal air impregnation, emptying of alveoli of polymer, and removal of xylene. Twice a day, the surface of the lungs and trachea was coated with a S10/S3 mixture, to assure a durable outer layer and to allow wicking of polymer into the lung parenchyma as the xylene evaporated from inside the lung. After 4 to 1O days of impregnation, the air source was removed and the lungs were coated with polymer and inverted for 24 hour periods, every day for one week.
At the end of this period, curing was commenced by bubbling air from the laboratory air supply through S6 and into the trachea for 15 minutes a day for three days. A plastic bag was placed around the lung preparation to serve as a gas curing chamber. The lungs were coated with polymer as needed and left bagged until cured.
All six percentages of S10/S3 polymer-xylene mix produced satisfactory specimens. Lungs impregnated with lower percentages of xylene were firmer and probably made better student specimens as they appeared to be more durable. Higher percentage xylene impregnated lungs were more flexible and spongy In texture. Hence, they may not be as durable, especially for student use. Canine and feline lungs were more flexible after impregnation than were porcine, ovine and bovine lungs. In all specimens, anatomical relationships were maintained (Fig.1).
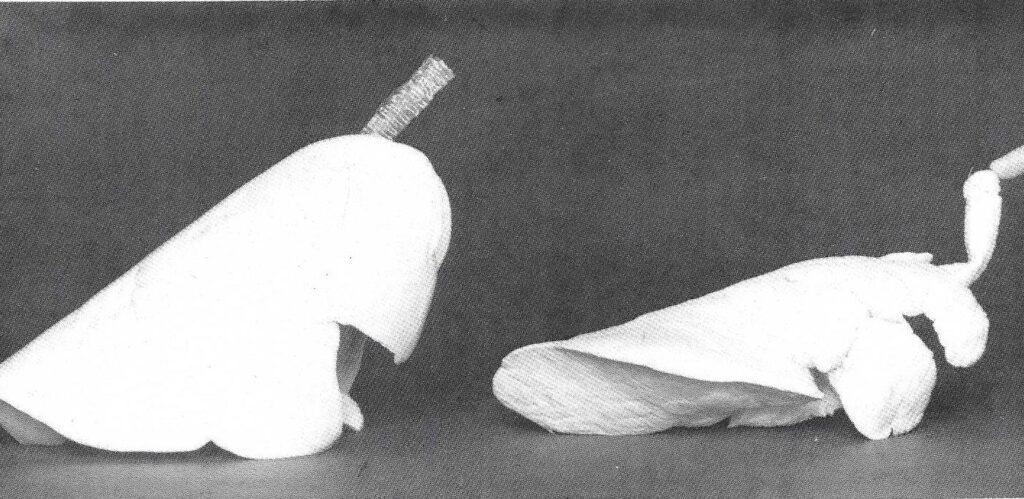
Figure 1: Right lateral view of canine lungs ,"forced air" Impregnated using 30% xylene/70% polymer-mix and porcine lungs · vacuum impregnated using 30% xylene/70% polymer-mix. Note anatomical relationships are more precise in the "forced air" impregnated lungs. However, the trachea of the vacuum Impregnated lung Is flexible and more durable.
Some specimens experienced areas of blow-outs during air drying and hence had areas which were not totally inflated. These lungs were not so beautiful, but still useful for teaching. Since air impregnation does not infiltrate dense tissues, the surface of the trachea must be coated as no polymer penetrates the dried cartilage. Hence, the trachea is hard and not flexible, whereas, vacuum Impregnated lungs yield a flexible trachea (Fig. 2).
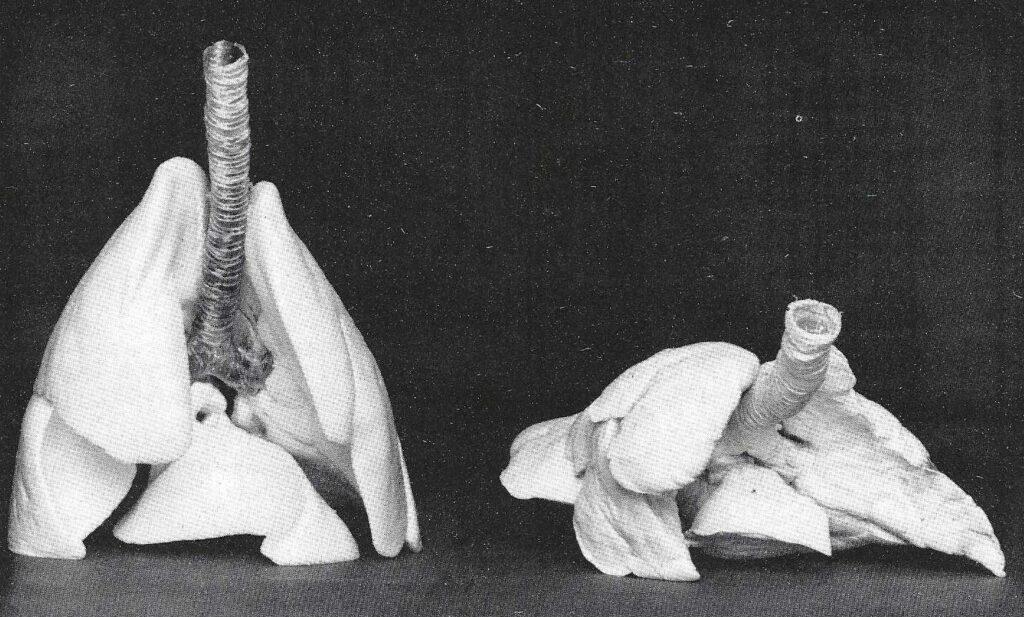
Figure 2: Cranial view of canine lungs (right), "forced air" Impregnated using 30% xylene/70% polymer-mix and porcine lungs (left), vacuum Impregnated using 30% xylene/70% polymer-mix. Anatomical relationships are not retained, as well as, In the vacuum Impregnated lungs.
All fat must be removed prior to air drying or the trachea will be greasy. Air impregnated lungs may not be as durable as vacuum impregnated lungs. However, the preservation of anatomical relationships is superior to vacuum impregnated lungs.
none