1Anatomia y Embriologia, Facultad de Veterinaria, Universidad de Murcia, Campus de Espinardo,30071 Murcia, Fax number: 34-68-36414 7,Murcia 30071. Spain.
2Departamento de Morfologia, Facultad de Veterinaria, Universidad de Las Palmas de Gran Canaria, Trasmontafla, Arucas 35416. Spain.
3Department of Comparative Medicine, College of Veterinary Medicine, University of Tennessee, 2407 River Drive, Knoxville, TN, 37996, USA.
Classically , the application of polyester polymer in plastination (P40 and P35, Biodur™) has been the production of brain or head slices. Recently semi-transparent body slices have been produced in our labs using P40 . No literature was found on the production of body slices using P40 . The purpose of this study was to develop a protocol for using P40 to produce slices from all regions of the body . Two-millimeter slices were cut from all regions of the body of a cat. The slices were processed using a modified P40 technique . Compared to the E12 method , the P40 technique offers not only comparable results, but also some extra advantages. Slices do not yellow; the method is easier for the beginner and an indefinite pot life of the impregnation bath. The P40 plastinated body slices yielded excellent anatomical detail of all tissues that were observed . In addition, they have been excellent aids for teaching and research in our classrooms and laboratories .
plastination; polyester; P40, sheet; section
R. Latorre: Telephone: 34 - 968 - 364 - 697; Fax: 34 - 968 - 364 - 147; e-mail: latorre@urn.es
R.W. Henry: Telephone: 865 - 974 - 5822; Fax: 865 - 974 - 5640; e-mail: rhenry@utk.edu
![]()



Classically the epoxy E12 (Biodur™) method has been used to produce body slices (von Hagens, 1979; 1985; von Hagens et al., 1987; Weber and Henry , 1993). However, the epoxy method has two main limitations : 1. Short period of time available to cast the impregnated slices (von Hagens, 1985; Latorre et al., 2002 a, b; Reed et al., 2002) and 2. Yellowing of E12 plastinated slices (Latorre et al., 2002a). P40 is used routinely for preserving brain sections (von Hagens, 1994; Barnett, 1997a, b; Henry, 1998; Sora et al., 1998a; Weiglein and Feig!, 1998; Henry and Weiglein 1999; Sora et al., 1999) and one use was for production of three-dimensional peripheral nervous tissue specimens, the brachia! plexus (Sora, 1998; Sora et al., 1998b). Some authors suggest that P40 may also be used for production of transparent body slices (Barnett, 1997b; Weiglein and Feig!, 1998) but no protocol or examples were given. However , using P40 for producing body slices (other than head slices) has not been reported until recently when Latorre and colleagues (2002b) prepared equine tarsal joint slices using P40.
In this work, a technique is described to produce slices of any portion of the body using P40 . Hence P40 is a viable alternative to the E12 method, for preserving semitransparent sections of the body.
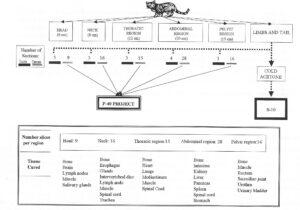
Figure 1. Regional schema of transverse slices indicating number of slices plastinated by both techniques (silicone - lcm; polyester - 2mm) after cold acetone dehydration and chart indicating tissue cured in each region.
An unembalmed feline cadaver had the hair clipped, was cleaned and frozen at -70°C. After freezing, it was divided into five regions (head, neck, thorax, abdomen and pelvis) (Fig. 1). Serially, two transverse slices, two millimeters thick and one transverse slice, one centimeter thick were cut from each block in a repetitive .manner. The number of slices for each region is detailed in figure 1. Slices were placed on aluminum grids and sawdust was removed by submerging them in cold acetone and scraping or flushing with a stream of cold water prior to submersion in cold acetone. A modification of the P40 technique (von Hagens, 1994) was used to plastinate the 2mm sections. The lcm sections were Plastinated using the standard silicone technique (von Hagens, 1985; von Hagens et al., 1987; Henry and Nel, 1993; Weiglein and Henry , 1993).
Dehydration:
Dehydration was by freeze substitution. Cleaned slices on their grids were initially submerged in 90% acetone at -15°C. The approximate acetone to specimen ratio was 10:1. Two changes of 100% cold acetone were carried out at weekly intervals.
Forced Impregnation:
After dehydration, the slices were impregnated using P40 (Biodur®) at room temperature. The specimens and grids were immersed into P40 polymer with no hardener or additives. After 24 hours, the immersed specimens and grids were placed in the vacuum chamber and pressure was decreased over a 24 hour period to 1Omm Hg while maintaining a rapid boil. Vacuum was held at 1Omm Hg for 5 hours until bubbling had greatly diminished . Vacuum was released and the submerged specimens returned to ambience. During the entire period of using P40, the polymer and the polymer with the slices were protected from light by using covered dark containers and a dark cover on the vacuum chamber. The container with the impregnated slices was returned to the freezer (-15°C) to hold until casting.
Casting and curing:
Flat chambers were made from 2 sheets of 2 or 3mm tempered (hardened) glass, 6mm silicone gasket (HS06- l Biodur™) and fold back clamps. Each impregnated slice was removed from the impregnation bath and placed in a flat chamber . Two ball bearings (3mm) were inserted into each chamber. The chambers were filled with fresh P-40, the gasket closed across the top of the flat chamber, and sealed with Biodur sealant (HS 80 Biodur™). The slices were centered in the sealed flat chambers by using a magnet to push the bearings against the slice (Barnett, 1997a) thus pushing the slice to the desired position . The bearings were parked at the perimeter by the gasket. The slices were laid flat (horizontal) on a supporting rack between two UV-A light units . Each UV light unit had 2 x 40 watt bulbs such that 2 bulbs were on each side of the flat chamber. The UV light serves as the catalyst for polymerization of the polyester polymer . To avoid excess heat build up during the curing phase, a thermostatically controlled switch was used to switch off the UV-A lights when the surface of the chambers reached 30°C. A fan (ventilator) was used to blow air continually over the chambers to cool them. Curing time was 30-60 minutes. After curing was completed, the clamps and the gaskets were removed and the glass chambers dismantled . The cast slices were wrapped in foil and the excess polyester was trimmed from the edges using a band saw.
The manufactured thin (2mm) tissue slices were semi-transparent and yielded excellent anatomical detail (Figs. 2, 3, 4, 5, 6, 7, 8). After curing, the polyester around the tissue was transparent and no yellowing was detected in any slices two and one half years later. The fat tissue was semi-transparent, and the other tissues or organs were significantly highlighted against the cleared fat. There were no problems impregnating or curing any type of tissue . All of the slices hardened after curing, even when the surface of the tissue slice was close to the glass of the flat chamber.
All P40 and silicone (Fig. 9) plastinated body slices yielded excellent anatomical detail of all regions of the body that were observed. The main organs and tissues checked in each region were the following :
Head slices: bone , brain, lymph nodes, muscle, salivary glands.
Neck slices: bone, esophagus, glands, intervertebral disc, lymph nodes, muscle, spinal cord, trachea.
Thoracic region: bone, heart, lungs, mediastinum , muscle, spinal cord.
Abdominal slices: bone, intestine, kidney, liver, pancreas , spleen, stomach, spinal cord.
Pelvic slices: bone, muscle , rectum, sacroiliac joint, urethra, urinary bladder.
These slices have been excellent teaching aids in our class rooms and aids in our research and laboratories.
The cast tissue slices provided a high degree of detail and permitted visualization of the various body structures in the normal topography of the region . Also the semi-transparency of the specimens allows viewing at the submacroscopic level. These characteristics are similar to those described for tissue slices produced by the E12 technique (Entius et al., 1993; Cook, 1997).
Methylene chloride was not used as an additional step for the lipid extraction. Even so, much of the fat tissue was semi-transparent in the polyester slices. This was likely due to the thinness of the slices, 2mm, which allowed much of the lipid to be extracted in the cold during the two-week dehydration . Similar to recent literature, impregnation used only P40 polymer (von Hagens, 1994; Barnett, 1997a; Sora, 1998; Latorre et al., 2002b) and no A4 hardener was added as currently recommended (Henry, 1998). By using no hardener or additives with the polyester, there is no time limit for casting the impregnated slices. Also the impregnation solution may be reused for another impregnation . With respect to the impregnation temperature, Sora and coworkers (1998, 1999) found that slices processed at - 25°C showed 2.48% less shrinkage than slices impregnated at room temperature. For such an anatomical project as this, the percent shrinkage is deemed small enough to use room temperature impregnation as other authors have (von Hagens, 1994; Barnett, 1997a; Sora, 1998a; Sora et al., 1999; Latorre et al., 2002b) rather than the more cumbersome cold impregnation (von Hagens, 1994; Sora et al., 1999). The lowered pressure used in this work was similar to that used by various authors (von Hagens, 1994; Barnett 1997a; Sora, 1998; Latorre et al., 2002b). Pressure was not allowed to go lower than 10mm Hg, to avoid extraction of monomeric styrene from the impregnation bath polymer (von Hagens, 1994). Our results show that it is not necessary to use the hardener, A4, within the casting solution for any area of the body. This is similar to the results of Barnett (1997a), Sora (1998), Sora and colleagues ( 1999), and Latorre and co-workers (2002b). Upon introduction of P40 to plastination, only UV-A light was used to cure the polyester . After a few years , a hardener, A4, was introduced to assure curing of P40 polymer in dense, dark tissues that might not cure uniformly using only UV-A light as the catalyst. Using this chemical activator within the casting solution has produced similar results (Henry, 1998). Both methods have continued to yield acceptable manufactured sheets for several years. The main advantage of using no A4 in the impregnation bath is that both the impregnation time and the casting time may be prolonged indefinitely . For this reason, we chose not to use the A4 hardener . The reported exposure time to UV-A light for curing is variable from 15 minutes to two days [15 minutes (Sora, 1998), 3 hours (Sora et al., 1999), 45 minutes to 4 hours (Weiglein and Feigl, 1998), 5 hours (Sora, 1998), 2 days (Barnett, 1997a). Weiglein and Feigl (1998) suggest that exposure time depends on wattage and on distance of the UV-A lights. Our exposure of 45 minutes of light from four 40-watt UV-A tubes at a distance of 20cm provided adequate exposure time for curing. The problems during light curing (too rapid curing and excess heat build up) reported by other authors (Weiglein and Feigl, 1998) were not encountered. Also the orange spots found in the cortex of the brain slices by others authors (Barnett, 1997a; Henry, 1998; Weiglein and Feigl, 1998) did not appear in our slices.
Compared to the E12 technique the advantages and disadvantages of the P40 technique are:
Advantages:
Disadvantages:
The results of this work demonstrate that the P-40 method (Biodur™) may be used to produce semi transparent body slices from any region of the body as with the E12 method (Biodur™). Furthermore, using P40 eliminates the disadvantages associated with the classic E12 technique .
Acknowledgments: This work was supported by Seneca Foundation , Comunidad Autonoma de la Region de Murcia (project number: PC/2/FS/99 and the grant: EE00789/CV/02) . The authors wish to thank Mr. Mariano Orenes and Ms. Helena Abellan of Murcia University for their excellent technical expertise in preparing the material.
Barnett RJ. 1997a: Plastination of coronal and horizontal brain slices using the P40 technique. J Int Soc Plastination 12(1):33-36.
https://doi.org/10.56507/YJVS5787
Barnett RJ. 1997b: The P40 technique scaled down. Abstract presented at The 5th Interim Meeting on Plastination University of Tennessee, Knoxville, TN, USA. June/July 1997.J Int Soc Plastination 12(2):36.
Cook P. 1997: Sheet plastination as a clinically based teaching aid at the University of Auckland . Acta Anat 158(1):33-36.
https://doi.org/10.1159/000147907
Entius CAC, Kuiper JW, Koops W, Gast A. 1993: A new positioning technique for comparing sectional anatomy of the shoulder with sectional diagnostic modalities: Magnetic Resonance Imaging (MRI), Computed Tomography (CT) and Ultrasound (US). J Int Soc Plastination 7:23-26. ·
https://doi.org/10.56507/MIIH4716
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S 10 method . J Int Soc Plastination 7(1):27-31.
https://doi.org/10.56507/WUXP9436
Henry RW. 1998: Update on polyester plastination (P40)! Where have all the "orange spots" gone? Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J Int Soc Plastination 13(2):30.
Henry RW, Weiglein A. 1999: Sheet plastination. of brain slices - P40 procedure. Abstracts presented at The 6th Interim Conference on Plastination, Rochester, New York, USA, July 11-16, 1999. J Int Soc Plastmabon 14(2):32.
Latorre RM, Reed RB, Gil F, Lopez-Albors 0, Ayala MD, Martinez-Gomariz F, Henry R. 2002a: Epoxy impregnation without hardener : to decrease yellowing, to delay casting and to aid bubble removal. J Int Soc Plastination 17:17-22.
https://doi.org/10.56507/LZKY8224
Latorre R, Vazquez JM, Gil F, Ramirez G, Lopez Albors 0, Ayala M, Arencibia A. 2002b: Anatomy of the equine tarsus : A study by MRI and macroscopic plastinated sections (S 10 and P40).Abstract presented at The 11th International Conference on Plastination, San Juan, Puerto Rico, July 14-19, 2002. J Int Soc Plastination 17:6.
Latorre RM, Reed RB, Henry RW. 2002c: Epoxy impregnation with no hardener. Abstract presented at The 11th International Conference on Plastination, San Juan, Puerto Rico, July 14-19, 2002. J Int Soc Plastination 17:7.
Reed RB, Henry RW. 2002: Epoxy under vacuum. Abstract presented at The 11th International Conference on Plastination, San Juan, Puerto Rico, July 14-19, 2002. J Int Soc Plastination 17:8.
Sora MC. 1998: Plastination of three dimensional brachia} plexus with P40 . J Int Soc Plastination 13(1): 12-14.
https://doi.org/10.56507/REZM6562
Sora MC, Brugger P, Bareck J, Motoc A. 1998a: P-40 plastination of human brain slices: Comparison between different immersion and impregnation conditions. Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J Int Soc Plastination 13(2):32.
Sora MC, Brugger P, Traxler H. 1998b: Plastination of three dimensional structures with P40. Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J Int Soc Plastination 13(2):32.
Sora MC, Brugger P, Traxler H. 1999: P40 plastination of human brain slices: Comparison between different immersion and impregnation conditions. J Int Soc Plastination 14(1):22-24.
https://doi.org/10.56507/XLSJ5724
von Hagens G. 1979: Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 194:247-256.
https://doi.org/10.1002/ar.1091940206
von Hagens G. 1985: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Heidelberg, Germany: Anatomisches Institut 1, Universitat Heidelberg.
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4):411-421.
https://doi.org/10.1007/BF00309677
von Hagens G. 1994: Plastination of brain slices according to the P40 procedure. A step-by-step description. 23 pages.
https://doi.org/10.56507/OWYV2878
Weber W, Henry RW. 1993: Sheet plastination of body slices - El2 technique, filling method. J Int Soc Plastination 7(1): 16-22.
https://doi.org/10.56507/EZGX2343
Weiglein A, Henry RW. 1993: Curing (Hardening, Polymerization) of the polymer - Biodur SlO. J Int Soc Plastination 7(1):32-35.
https://doi.org/10.56507/ABNZ7085
Weiglein AH, Feigl G. 1998: Sheet plastination of brain slices according to the P35 and P40 procedures. Abstract presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998. J Int Soc Plastination 13(2):30.