1 Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort 0110, Republic of South Africa. email marthinus.hartman@up.ac.za
2 Department of Anatomy and Physiology, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort 0110, Republic of South Africa.
A technique to demonstrate the arterial supply of the female reproductive organs of the African lion (Panthera leo) is described. A 122 kg, one year old nulliparous lioness was used. A tin-condensation based, room temperature vulcanization silicone with a durometer shore 30 A hardness silicone, coloured with red pigment was used to fill the reproductive arterial supply in situ via the abdominal aorta. After the silicone polymer-mix cured, the organs were removed and macerated yielding a flexible arterial cast of the female reproductive organs. The cast endured handling well and showed good flexibility. In situ casting has the advantage of minimal leakage since no vessels are cut in the system to be studied.
African lion; anatomy; arterial supply; silicone cast
Marthinus Hartman Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria, Private Bag X04, Onderstepoort 0110, Republic of South Africa. email marthinus.hartman@up.ac.za
![]()



The surplus of African lions on lion farms in South Africa has created a need for population control. No description of the vascular supply of the female African lion’s (Panthera leo) reproductive organs was found in the literature and its accurate anatomical detail had to be established. Cast production, to aid anatomical description, dates to the late fifteenth century. At this time Leonardo da Vinci used wax to inject the ventricles of the sheep brain (Huard, 1968). Since then various products have been used to aid in anatomical descriptions. The preparation of organ silicone casts to study the trachea and bronchi (Henry, 1992a) and its associated vasculature (Henry, 1992b) and the chambers of the heart and its great vessels (Henry et al., 1998) has been done since 1992. In 1995 a resin cast with E20 red was used to study anatomical cavities (Pretorius and Geyer, 1995) and the comparative use of gelatine and silicone in brain ventricles was done in 2000 (Grondin, 2000). Resin, latex and acrylic casts have been illustrated in a Veterinary textbook (König and Liebich, 2004).
Improvements on existing techniques have been evaluated recently. A more comprehensive study to enhance the quality and durability of silicone specimens of the human heart, tracheobronchial tree and brain ventricles was done (Aultman et al., 2003). In this study, a process describing four stages was advocated that included harvesting and preparation of the organs, silicone injection, curing and maceration. The sequence in which plastination chemicals are added can improve stability of the silicone impregnation mixture at room temperature (Glover, 2004) and cold-temperature techniques have been used commonly over the past 20 years (De Jong and Henry, 2007). Silicone casting of the uterine lumen of a mare has also been reported (Chaurasia and Nayak, 2009) and the preservation of organs using an alkyd resin has been used in various species (Ari and Çinaroğlu, 2011).
In order to perform laparoscopic sterilization of the African lioness (Panthera leo) a sound knowledge of the morphology and arterial supply of the female reproductive tract is required. A morphological study of the splanchnology and topography of the female reproductive organs of the African lion has already been performed by the authors on three cadaveric specimens (Hartman and Groenewald, 2012) however detailed knowledge of the arterial supply was lacking. In situ vascular silicone casting was chosen for the purpose of studying the female lion’s reproductive blood supply prior to performing any surgical procedure on this organ system. A local company in South Africa was sourced to supply silicone materials, red pigment and equipment (Advanced Materials Technology (Pty) Ltd, 2010).
A one year and one month old captive bred nulliparous lioness weighing 122.0 kg was used. She was initially immobilized with 360 mg of a tiletamine/zolazepam combination (Zoletil®, Virbac, South Africa) administered via a 3ml remote injection dart (DanInject®, South Africa). An 18G intravenous catheter (Jelco®, Smiths Medical) was placed in a lateral saphenous vein and an additional 140 mg of Zoletil was given intravenously. The lioness was then transported to the Onderstepoort Veterinary Academic Hospital (OVAH) under constant supervision.
Upon arrival at the OVAH a second 14G intravenous catheter (Jelco®, Smiths medical) was placed in one of the cephalic veins and 300mg of propofol (Propofol 1% Fresenius, Fresenius Kabi, South Africa) given intravenously. The lioness was intubated and maintained on isoflurane (Isofor, Safeline Pharmaceuticals Pty Ltd, Florida, South Africa). A third 14G intravenous catheter was paced in the external jugular vein and heparin sodium 5000IU/ml (Fresenius 5ml/vial) at a dose of 75 Units/kg (1.8ml) injected.
Procedure
The jugular vein was used to flush the entire cardiovascular system through with 3L Ringer’s lactate. After approximately 2L of Ringer’s lactate had run in a metal trocar and cannula was placed in the contralateral carotid artery by means of a cut down technique. While under anaesthesia the lioness was exsanguinated. Euthanasia was therefore effected without recovery from anaesthesia, while the patient was still in plane two of anaesthesia.
An incision was made through the abdominal wall at the lateral border of the rectus abdominis muscle. Two additional incisions extending from this paramedian incision to the lateral border of the epaxial muscles directly caudal to the thirteenth rib as well as cranial to the tensor fasciae latae muscle were completed. The lateral abdominal wall was reflected dorsally using this technique bilaterally. Since this was a fresh specimen without rigor mortis it was possible to reflect all the layers of the lateral abdominal wall dorsally in one layer. Because the caudal vena cava is situated to the right and ventral to the aorta, the aorta was approached from the left and was ligated immediately cranial to the renal arteries in order to secure preservation bilaterally of the origins of the ovarian arteries. The caudal mesenteric artery was ligated where it was readily accessible in the mesocolon to prevent silicone from being lost to the arterial supply to the large intestine. No other dissection was done in order not to lacerate any blood vessels which would allow extravasation of silicone product. The project was approved by the University of Pretoria Animal Use and Care Committee and Research Committee (protocol number v038-09). Due to the ethical sensitivity of wildlife research the use of larger number specimens was not feasible.
Preparation of the product
Mold Max 30 RTV silicone, durometer shore 30 A hardness, viscosity 25000cps (Advanced Materials Technology (Pty) Ltd, 2010, from Smooth-On, USA) which is a tin/condensation cure (rather than platinum/addition cure) was obtained. Five hundred grams of polymer were measured into a 1 kg plastic bucket and 5% by weight silicone thinning fluid was added to decrease the viscosity nearly 50% (13800cps) to enhance injection. To create a red color, 15 ml of red silicone pigment was added and mixed for 5 minutes; 10% (50 grams) parts by weight of MM30 STD catalyst was then added to the thinned and colored silicone and stirred thoroughly for 5 minutes. Once the catalyst is added, curing will begin within 60 minutes. This silicone preparation was placed into a 5L plastic bucket in preparation for degassing. The bucket with the thinned, coloured, polymer-mix was placed in a vacuum chamber and a vacuum of 0.86 bar (28mm Hg) applied. As full vacuum was approached, air bubbles rose to the surface from within the polymer. This trapped air expanded the volume of the mix ~ 4X, rising almost to the top of the bucket and then spontaneously collapsed. Vacuum was released and then immediately applied a second time. During the second vacuum cycle, the silicone did not rise to the same extent. The silicone preparation was poured slowly into a 400 ml twin-chamber cartridge from a one metre height to further eliminate pre-existing air bubbles. The dual chamber cartridge can be used to mix two different solutions, however in our study the silicone was already prepared. Two plungers were inserted into the cartridge without their black plastic seals to allow excess air to escape. The seals were inserted as soon as silicone appeared through the escape ports. The cartridge was inserted into the dispensing handgun and the static mixing nozzle was screwed on to the tip of the cartridge (Fig. 1).
The static mixing nozzle was filled with silicone-mix to expel air from the nozzle. The total silicone preparation time was 35 minutes.
The aorta was transected immediately caudal to the ligature that had been applied earlier, and the caudal part of the abdominal aorta was secured to the tip of the dispenser of the silicone gun using two 0 nylon ligatures (Fig. 2). Prepared, thinned and coloured silicone was injected by hand pressure until the silicone appeared in an incision made in the plantar aspect of the metatarsus. The injection device was left attached to the aorta to retain silicone intravascularly during the curing process.
The cadaver was stored overnight at 23°C which is ideal for curing. Approximately 400 ml of silicone-mix was used.
Maceration
After 21 hours of curing the silicone in a normal position, the organ system was removed from the cadaver. Ostectomies of the pelvic floor were performed at the lateral borders of the obturator foramina and through the lateral border of the ramus of the ischium to remove the floor of the pelvic cavity and the contents of the pelvic cavity and abdominal reproductive organs. The intra-pelvic arteries including the entire length of bilateral internal pudendal arteries were removed with the pelvic and reproductive viscera. The caudal gluteal artery was severed soon after the internal pudendal artery branched off. After removing the reproductive tract, the tissue block was placed into 10% potassium hydroxide (KOH) in distilled water. After six days of maceration (Fig. 3) the KOH was decanted and replaced with fresh 10% KOH. However, this new solution of KOH was made with tap water instead of distilled water. This new solution was ineffective due to precipitation of the KOH and resulted in the solution becoming milky and ineffective. A new KOH solution was constituted using distilled water. This solution once again was effective and showed no signs of precipitation. After two more weeks the KOH was replaced with fresh solution. At this stage (three weeks after the start of the maceration process) all the tissues were macerated apart from tissue around the vulva area which was originally very bulky.
After another two weeks of maceration (five weeks in total) some gelatine-like tissue was still present in the area of the vulva. However at this stage it was decided to place the casts in a running water bath to rinse off the remaining tissue. This had good results; the remaining tissue disintegrated into smaller pieces through the hydrodynamic forces of the running tap water within one hour. In areas with tissue remnants, gentle digital manipulation was applied and together with running water remaining tissue was removed. The casts were boiled in water for thirty minutes until all tissue remnants were denatured and removed. The casts were then placed in 6% hydrogen peroxide for two hours. Some very small remnants had to be gently teased off with forceps. The casts were left in a running water bath overnight to yield a completely macerated cast of the arterial system. The arteries were then allowed to re-arrange themselves in their natural position in a solution of glycerine, formalin and water which allowed the cast to be suspended inside a Perspex container built to size.
This process yielded a silicone cast of good quality in terms of detail, flexibility and anatomical accuracy (Fig. 4). The casts endured handling well due to good flexibility and appeared to provide a true replica of the arterial supply of the female reproductive organs of the African lion (Panthera leo).
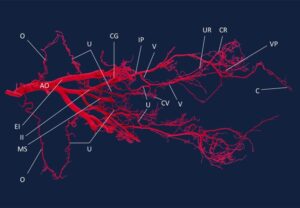
Fig. 4. Red pigmented silicone cast depicting the arterial supply to the reproductive organs of the African lioness. Aorta (AO), External iliac artery (EI), Internal iliac artery (II), Median sacral artery (MS), Ovarian artery (O), Uterine artery (U), Caudal gluteal artery (CG), Internal pudendal artery (IP), Vaginal artery (V), Caudal vesicular artery (CV), Urethral artery (UR), Caudal rectal artery (CR), Ventral perineal artery (VP), Clitoral artery (C).
The value of studying the arterial supply to an organ system in species of unknown anatomy using silicone casting in situ prior to surgical exploration provides valuable information for the surgeon.
We found that silicone can be used to a produce arterial casts of accurate anatomic detail similar to other techniques (König and Liebich, 2004). This is a study describing the use of a silicone product to study arterial supply to an organ system situated in a complex anatomical location as used in other studies (Smodlaka et al., 2008, 2009) in contrast to the use of loose organs (Henry, 1992b; Aultman et al., 2003).
The arterial casts were severely entangled by the running water in the water bath. It was challenging and time consuming to re-arrange the arteries in their natural orientation after this process. Leaving the casts in a running water bath might be regarded as an unnecessary step in the authors’ opinion. However once the casts were disentangled the silicone casts tended to adapt their own spatial arrangement by virtue of the memory in the silicone over time. The use of tap water in preparation of the KOH solution should be avoided and distilled water is essential. A firmer product such as resin might not result in this complication.
In this study a platinum / addition cure silicone was first used which suffered cure inhibition and did not cure properly inside the arteries. Cure inhibition can result on a variety of surfaces, sulphur being the biggest culprit, and biological tissues being the cause in our study. Tin / condensation cure silicones lose some moisture over time, shrink slightly, dry out and then become easier to tear. This process can take 1 - 6 years depending on the quality of the silicone (MM30 has a typical life of 5 years) (Advanced Materials Technology (Pty) Ltd., 2010) when after this point the silicone becomes more delicate to handle. Platinum / addition cure silicones however do not lose any moisture so the shrinkage is negligible; this silicone will have a much longer shelf life once cured. Current reports of platinum silicone moulds over 20 years old exist and they would probably last a lifetime (Advanced Materials Technology (Pty) Ltd., 2010).
This technique provided the ability to fill the entire arterial supply of the female reproductive organs in situ similar to the use of silicone in loose specimens (Aultman et al., 2003). It was possible to depict the spatial arrangement of the arteries of this visceral and intra-pelvic organ system in its natural position by then allowing the cast to cure inside the carcass. It was possible to remove the organ structure from a complex location in the body, complete the maceration process and yield a cast reflecting the arterial anatomy. Care has to be taken during dissection of the internal pudendal artery due to its complex anatomical location. This method prevents leakage of silicone from severed vessels should the organs be removed prior to casting, and it could possibly be used for other organ structures.The cast was of good quality in terms of detail and anatomical accuracy with the main arteries infiltrated. The cast endured handling well and showed good flexibility. Silicone can be used in abdominal organs in situ to illustrate, study and anatomically describe arterial supply. The arterial supply to the reproductive organs of the African lioness is similar to that of the domestic cat and information obtained from this silicone study could subsequently be used during laparoscopic ovariectomy and salpingectomy of the African lioness (Hartman et al., 2013). The results from this technique were also used in a subsequent study to compare the efficacy of direct observation and trans-illumination in describing the arterial supply to the reproductive organs of the African lioness.
Acknowledgements
The authors thank Mr. Paul Carnall of Advanced Materials Technology (Pty) Ltd., Kempton Park, South Africa for supplying equipment and technical assistance.
Advanced Materials Technology (Pty) Ltd., 2010. Mold Max 30. Kempton Park, South Africa.
Ari HH, Çinaroğlu S. 2011: A new approach to preservation of some organs using alkyd resin. Res Vet Sci 90: 16-19.
https://doi.org/10.1016/j.rvsc.2010.05.017
Aultman A, Blythe J, Sowder H, Trotter R, Raoof A. 2003: Enhancing the value of organ silicone casts in human gross anatomy education. J Int Soc Plastination 18: 9-13.
https://doi.org/10.56507/JPGL6406
Chaurasia S, Nayak V. 2009: Silicone corrosion cast of uterus of mare for museum. Vet Pract 10: 179-180.
De Jong K, Henry RW. 2007: Silicone plastination of biological tissue: Cold-temperature technique - Biodur S10/S15 technique and products. J Int Soc Plastination 22: 2-14.
https://doi.org/10.56507/ZLMJ7068
Glover R. 2004: Silicone plastination, room temperature methodology: Basic techniques, applications and benefits for the interested user. Abstract presented at The 12th International Conference on Plastination, Murcia, Spain July 11-16, 2004. J Int Soc Plastination 19: 7.
Grondin G, Sianothai A, Orly R. 2000: In situ ventricular casts of S10 plastinated human brains. J Int Soc Plastination 15: 18-21.
https://doi.org/10.56507/APKG6089
Hartman MJ, Groenewald HB. 2013: Morphology of the female reproductive organs of the African lion (Panthera leo). Acta Zool-Stockholm 94: 437-446.
https://doi.org/10.1111/j.1463-6395.2012.00572.x
Hartman MJ, Monnet E, Kirberger RM. 2013: Laparoscopic Sterilization of the African Lioness (Panthera leo). Vet Surg 42: 559-564.
https://doi.org/10.1111/j.1532-950X.2012.01049.x
Henry RW. 1992a: Silicone tracheobroncial casts. J Int Soc Plastination 6: 38-40.
https://doi.org/10.56507/LOVB7516
Henry RW. 1992b: Silicone pulmonary vascular casts with attached tracheobronchial casts. J Int Soc Plastination 6: 41-44.
https://doi.org/10.56507/MKMC2434
Henry RW, Daniel GB, Reed RB. 1998: Silicone castings of the chambers of the heart and the great vessels. J Int Soc Plastination 13: 17-19.
https://doi.org/10.56507/AWGT3303
Huard P. 1968. Leonardo da Vinci. Dessins anatomiques (anatomie artistique, descriptive et fonctionelle). Paris: Roger Dacosta.
König HE, Liebich HG. 2004: Veterinary anatomy of domestic mammals. Schattauer, Stuttgart, New York.
Pretorius WF, Geyer HJ. 1995: The use of E20 red resin for casting anatomical cavities. J Int Soc Plastination 9: 37.
https://doi.org/10.56507/JQTR9706
Smodlaka H, Henry RW, Schumacher J. 2008: Macroscopic anatomy of the heart of the ringed seal (Phoca hispida). Anat Histol Embryol 37: 30-35.
https://doi.org/10.1111/j.1439-0264.2007.00791.x
Smodlaka H, Henry RW, Reed RB. 2009: Macroscopic anatomy of the ringed seal [Pusa (phoca) hispida] lower respiratory system Anat Histol Embryol 38: 177-183
https://doi.org/10.1111/j.1439-0264.2008.00904.x