University Sts Cyril and Methodius, Faculty of Veterinary Medicine. Department of Functional Morphology, Lazar Pop Trajkov 5-7, 1000 Skopje, Macedonia, Europe
Kidneys from five month-old mixed-breed pigs were collected and 2-3mm thick longitudinal slices were prepared for viewing sub-gross anatomy as fresh tissue or as routinely plastinated tissue with and without degreasing. Standard cold silicone plastination procedures were used. Sliced fresh tissue and cured plastinated specimens were placed on a glass plate and back-lit to evaluate anatomical detail. All specimens yielded similar anatomical detail. However, degreased, plastinated specimens yielded the most anatomical detail. These thin silicone slices produce a durable permanent record similar to epoxy sections without the need for casting slices.
plastination; silicone; porcine; kidney; tissue
![]()



Plastination of tissue by polymer impregnation is a unique method for preserving specimens in a permanent way (von Hagens, 1986). Sheet plastination is an internationally accepted method for preservation of tissue slices (Weber and Henry, 1993; Sora and Cook, 2007; Weber et al., 2007). It has been shown that sheet plastinated slices are excellent tools for demonstrating the anatomical topography of structures within specimens. The E12 and P35/40 techniques have become the methods of choice for creating 2-5mm or even 8mm semitransparent organ or body slices (Sora and Cook, 2007; Latorre and Henry, 2007). These techniques offer a unique opportunity for radiographic- anatomic-pathologic correlation and facilitation of understanding of complex anatomical relationships (Mc Niesh, 1988).
The S10 technique is best known for preservation of isolated organs or whole bodies (Henry, 1997; de Jong and Henry, 2007). This technique is also used for producing thick slices (0.5 – 1.0cm or more) which are utilized widely for education (von Hagens, 1987; Weiglein, 1997). S10 plastination of entire or halved kidneys is common (von Hagens, 1986; Oostrom, 1998; Ilieski, 2005; Pereira-Sampaio et al., 2007). However, no studies were found in which thin slices of kidneys were prepared and plastinated using the cold silicone S10 process.
Therefore, in order to visualize sub-gross structures of the kidney for anatomical study and research, a protocol was developed which could use the S10 method for producing thin sheet plastinated kidney slices.
A total of 60 kidneys from five month-old pigs weighing 95kg (mean) were collected from two breeds at the abattoir for this study. Thirty kidneys were from mixed-breed Dalland pigs and 30 kidneys were from mixed-breed Landrace/Yorkshire pigs. The kidneys were removed from the pig carcasses together with the surrounding adipose tissue in order to preserve kidney shape and size. Upon arrival at the laboratory, the renal fat and capsule were removed and the kidneys were
flushed in cold tap water for three hours. The kidneys were then cooled in a refrigerator at +4oC for two hours to firm the tissue for slicing 2-3mm thick longitudinal sections.
Specimen preparation, dehydration and defatting
Tissue slices were cut on a deli slicer (Fig. 1) and divided into three groups: 1. For plastination after fixation and degreasing, 2. For
plastination after fixation but without degreasing, and 3. For viewing as fresh tissue (no fixation or plastination).
Group 1. Each slice for plastination was numbered and placed on a wire mesh (Fig. 2). Another wire mesh was placed on top of the slice forming a sandwich of wire mesh/specimen slice/wire mesh/specimen slice/wire mesh, etc. (Sora and Cook, 2007; Henry and Latorre, 2007). The stack of sandwiched slices was placed in a plastic box and the slices were rinsed with flowing cold tap water for one hour to remove remaining blood (Weiglein, 1997; Sora and Cook, 2007). After flushing, the stacked slices were submerged in 3% formalin solution for five days for fixation (Oostrom, 1987). After fixation the sandwiched slices were transferred into in a stainless steel basket and were rinsed with cold tap water overnight to flush out the formalin. Before dehydration, the slices and water were pre-cooled in a refrigerator (+5oC) for five hours. Dehydration of specimens was carried out using the freeze substitution method in pure, cold (-25oC) acetone with a
fluid:tissue ratio of 10:1 (Tiedemann, 1988). The basket with slices was removed from the water bath and submerged in the first 100% acetone bath for five days. The slices were then transferred into the second acetone bath for another five days. After transfer of the slices into the third acetone bath for five days, acetone concentration was monitored to make sure that the final acetone percentage was at least 99% for three more days. Acetone purity was monitored with an acetonometer (de Jong and Henry, 2007). After complete dehydration of specimens, the final acetone bath was allowed to gradually warm to ambient (room) temperature for three days to hasten degreasing of the specimens.
Group 2. Slices were produced to compare the clarity of anatomical structures of plastinated pig kidney slices to that of plastinated slices which had not been degreased prior to impregnation. This protocol was the same as for the degreased slices, except they were impregnated when dehydration was complete and not brought out to degrease at ambient temperature. The slices were placed directly into the impregnation polymer from the cold acetone. Impregnation and curing were identical to the degreased plastinated slices.
Group 3. Slices were produced to observe and compare their anatomical structure as fresh tissue slices only and were not plastinated. Their initial preparatory steps were identical to those of slices to be plastinated. However, after the one hour flush to remove any remaining blood and before fixation, their anatomical features were examined using bright, back light illumination similar to viewing of the plastinated slices.
Impregnation and curing
Cold impregnation of kidney slices with the silicone reaction-mixture was carried out by continuous impregnation as established by Dr. von Hagens (1986). The dehydrated slices were immersed in a mixture of silicone polymer and catalyst [containing a chain extender (S10/S3)] at a ratio of 100:0.5 and allowed to sit and equilibrate in the -20°C polymer-mix for three days.
Thereafter, vacuum was applied and pressure was slowly decreased to ~8mmHg over 10 days. The rate of pressure decrease was monitored by observing bubble formation on the polymer surface and then setting the parameters daily for the digital vacuum controller (Fig. 3). The controller consists of a digital manometer and needle valve for pressure increase (open the valve) or pressure decrease (close the valve). The vacuum controller semi-automatically decreases pressure once it has been programmed for that incremental decrease. Prior to a decrease of pressure, the controller will allow an increase in pressure of 10mmHg. This increase in pressure allows tissues to relax and thus release the vaporized acetone which in turn allows better uptake of the S10/S3 mixture into the tissue and hence minimizes shrinkage. Then the controller automatically decreases pressure to the set level to
maintain bubble formation and acetone vaporization. The pressure parameters are set daily in conjunction with observation of bubble production.
Impregnation was judged complete when bubble production ceased and pressure was stabilized at ~8mmHg. After impregnation, the vacuum chamber and its contents were removed from the deep freezer and placed at room temperature. The pressure was slowly increased to atmospheric pressure over a three day period (Henry and Nel, 1993; deJong and Henry, 2007).
The impregnated slices and grids were removed from the plastination kettle as a unit and the excess coating of polymer was allowed to drain from the slices and screen (Fig. 4). After draining, the slices were placed on paper towels and covered with towels for 24 hours to continue the removal of excess surface silicone (Fig. 5).
Curing was carried out in a gas curing chamber in which the specimens were exposed to
S6 vapors for five days at room temperature (Fig. 6). An aliquot of CaCl2 was placed in the chamber to control moisture. A small membrane pump was used to bubble air through the S6 to enhance vaporization of the liquid gas cure and hence accelerate curing of the kidney slices (Weiglein and Henry, 1993; de Jong and Henry, 2007). After five days exposure to S6, curing of the kidney slices was complete.
Slice evaluation
Each slice was placed on a clear glass plate, at the intended time, and examined and photographed using the background light (epidiascope) to illuminate the kidney from beneath.
A steps and timetable summary for the S10- technique for thin kidney slices is as follows:
SLICE: cold, non-fixed kidneys FLUSH; 1 hour cold tap water
FIX: 5 days room temperature formalin (3%) FLUSH: overnight cold tap water
COOL: 5 hour at +5°C DEHYDRATE: 15 days at -25°C
DEGREASE: 3 days at room temperature POLYMER IMMERSION: 3 days at -20°C FORCED IMPREGNATION: 10 days at -20°C
POST-IMPREGNATION: 3 days at room temperature GAS-CURING: 5 days at room temperature
Thin slices of the kidney were produced using a modified S10 plastination protocol along with and without degreasing. The degreased plastinated slices were of good quality and semitransparent. Plastinated slices yielded clarity of sub-gross anatomy. They were thin and semitransparent and fine detail of many anatomical structures could be observed. The slices were flexible, dry, and odorless with smooth surfaces, were easy to handle and to evaluate. They were prepared with relative ease and limited expense and are suitable for storage at room temperature. Neither noticeable shrinkage nor distortions were observed in the slices.
Anatomic detail down to the wall and lumen of interlobar arteries (a. interlobaris) was clearly delineated from the surrounding
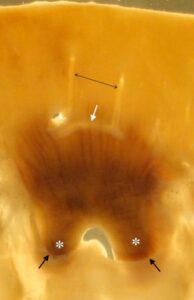
Figure 10. Anatomical detail of degreased, plastinated pig kidney slice. Arcuate artery (white arrow), interlobular arteries (black, double headed arrow), perimeter of calyx (black arrows), renal papillae (*).
kidney tissue (Figs. 7, 8, 9) as were the arcuate arteries (a. arcuate) (Fig. 10). Similarly, most sub-gross anatomy of the plastinated S10 pig kidney slices was easily recognized. Distinction between renal cortical structures and renal medullary structures was evident (Figs. 7, 9). The renal cortex was light colored and the renal medulla, represented by pyramids, was dark in color. The renal pyramids were triangular in form with discrete radial structures and their base was directed toward the outer cortical surfaces of the kidney (Fig. 7). The conical renal papilla of a pyramid was surrounded by a renal calyx that was cup shaped (Fig. 7).
Fresh tissue slices had wet surfaces and their anatomical subdivisions were loose and difficult to handle and evaluate. Hand manipulation of these specimens was difficult since the component relationships were easily distorted or disrupted. With background lighting, the medullary structures were reddish colored with no clear demarcation from surrounding lighter colored cortical tissue (Fig. 11).
The dehydrated but not degreased slices showed distinction between the renal structures. However, finer details such as the lumen of some segmental arteries within the parenchyma were not clearly delineated. These slices were less transparent with less delineation between the blood vessels inside the kidney parenchyma primarily due to the presence of fat (Fig. 12).
The dehydrated and degreased slices showed clear distinctions between the renal structures down to the finer detail of sub-gross structures (Fig. 13)
Our goal of producing thin slices of the kidney using a modified S10 plastination protocol which would aid anatomical study was successful. Clarity of sub-gross anatomy was similar to specimens produced by the E12 or P35/40 methods. This is primarily due to the thinness of slices and resultant transparency which yielded anatomical detail of many structures.
To date, epoxy and polyester plastinated slices have no flexibility and are brittle and often fracture if dropped. Classically, silicone plastinated specimens have a degree of flexibility. To provide maximal flexibility of kidney slices, only one half of the S3 catalyst was used to make the reaction-mixture. This along with slice thinness assured that the silicone slices had good flexibility.
As was expected, shrinkage was minimized by cold dehydration. Allowing the slices to equilibrate both when loaded into the reaction-mixture and upon conclusion of impregnation before decreasing or increasing pressure respectively is also known to minimize tissue shrinkage. In addition, the planned incremental decreases of pressure by the vacuum controller, allow uniform decreases of pressure until pressure is reduced to 8mmHg. This uniform decrease of pressure along with alternate timed 10mmHg increases in pressure allows tissues to relax and thus release the vaporized acetone which in turn allows better intake of the S10/S3 mixture into the tissue and hence minimizes shrinkage.
Cook (1997) reported that E12 plastinated kidney sections when viewed in situ body slices yielded an informative profile of the capsule, cortex, medulla and pyramids. Similarly, the sub-gross anatomy of the plastinated S10 pig kidney slices was easily recognized and apparently observed in yet more detail.
One of the main features of E12 or P35/40 specimens is the transparency of the specimen slice (Steinke, 2002; Sora and Cook, 2007; Weber et al., 2007: Henry and Latorre, 2007). Bringing the last acetone bath to room temperature for a few days produced a satisfactory degree of transparency in S10 slices by reducing the fat accumulation in the renal sinus. Likely more transparency of the renal sinus could be obtained by a few more days of room temperature acetone defeating. However, the dehydrated but not degreased slices and the fresh slices both showed less transparency and hence less distinction between renal structures. Medullary structures were dark colored and could be recognized from the surrounding cortical tissue yet segmental arteries in the parenchyma and their lumina were not clearly delineated from the surrounding kidney tissue due to the high lipid content of the renal sinus. This work demonstrates that thin S10 semitransparent kidney slices can contribute to future research activities. The aim of our next study will be to explore the segmental arterial structure in pig kidney.
Being able to visualize the sub-gross anatomy in situ on the thin S10 plastinated slices, should have a positive effect in undergraduate and postgraduate teaching. Students provided with sagittal sections of the kidney can visualize a complete overview of renal anatomy. Besides the great educational value that S10 thin specimens will have, these kidney slices for a research investigation. As well, the possibilities of three dimensional reconstruction of the thin plastinated kidney will allow many opportunities for further investigation, one being counting of the number of renal pyramids per kidney in both breeds and to analyze the variations in the way they unite and way they open into the renal pelvis.
The degreased S10 plastinated pig kidney slices prove to be an excellent teaching and research tool in anatomy. The kidneys plastinated in thin slices by this technique are safe for student handling and use. Students can handle these slices and reconstruct the pig kidney. This will aid their understanding of the specific anatomical detail of the 2-3mm thick specimens; as well as help them bridge the gap between gross and histological structures.
Due to the possibility for analysis of the space relationship between the renal papilla inside the renal calyx (Fig. 12), the thin plastinated slices will be used for future research activities in endourology where a three dimensional view may aid locating kidney stones inside the lumen of the calyx. We believe this will contribute to development of new or a modification of available techniques for stone disease treatment. The knowledge of anatomy based on thin plastinated kidney slices will also assure more accurate interpretation of diagnostic CT or MR scans.
We can conclude that the S10 technique may be used for producing 2-3mm organ slices. As well, sub- gross anatomy is distinct and these slices are a better aid from which to study and record the various aspects of anatomy in the kidney than fresh slices or non- degreased slices. The method that we applied is easy to follow and uses materials that are found in the most basic plastination laboratory.
Cook P, Al-Ali S. 1997: Submacroscopic interpretation of human sectional anatomy using plastinated E12 sections. J Int Soc Plastination 12(2):17-27.
https://doi.org/10.56507/XICY2283
De Jong K and Henry RW. 2007: Silicone plastination of Biological Tissue: Cold temperature Technique Biodur S10/S15 Technique and Products. J Int Soc Plastination 22:2-14.
https://doi.org/10.56507/ZLMJ7068
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7(1): 27-31.
https://doi.org/10.56507/WUXP9436
Henry RW, Janick L, Henry C. 1997: Specimen preparation for silicone plastination. J Int Soc Plastination. 12(1): 13-17.
https://doi.org/10.56507/HVSK9838
Henry RW, Latorre R. 2007: Polyester Plastination of Biological Tissue: P40 technique for Brain Slices. J Int Soc Plastination 22:59-68.
https://doi.org/10.56507/VKCL2525
Ilieski V, Pendovski L, Ulcar I. 2005: Evaluation of Shrinkage on Pig Kidneys with S10 technique: Study before and After Plastination. Proceedings of the 8th Interim Conference for Plastination, 42-43.
Latorre R, Henry RW. 2007: Polyester Plastination of Biological Tissue: P40 technique for Body Slices. J Int Soc Plastination 22:69-77.
https://doi.org/10.56507/CARV3913
McNiesh LM, von Hagens G. 1998: The diagnostic imaging characteristics of plastinated anatomical specimens. J Int Soc Plastination 2(1):24-39.
https://doi.org/10.56507/RMEK8272
Oostrom K. 1987: Fixation of tissue for plastination: General Principles J Int Soc Plastination 1(1):3-11.
https://doi.org/10.56507/WLZH2223
Oostrom K. 1998: Plastination of the human kidney. J. Int. Soc. Plastination 2(2):21-24.
https://doi.org/10.56507/DCIG8245
Pereira-Sampaio MA, LA Favorito, Henry RW, FJB Sampaio. 2007: Proportional analysis of pig kidney arterial segments: Differences from the human kidney. J Endourology: 21(7) 784-788.
https://doi.org/10.1089/end.2006.0318
Sora M-C and Cook P. 2007: Epoxy Plastination of Biological Tissue: E12 technique J Int Soc Plastination 22:31-39.
https://doi.org/10.56507/FCTY3173
Steinke H, Thomas M. 2002: Plastination: Correlation of anatomical specimens and MRI. Klinische Sportmedizin/Clinical Sports Medicine-Germany (KCS) 3(3):41-46.
Tiedemann K; Ivic-Matijas D, 1988: Dehydration of macroscopic specimens by freeze substitution in acetone. J Int Soc Plastination, (2): 2-12.
https://doi.org/10.56507/SCLL2742
von Hagens G. 1986: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomische Institut 1, Heidelberg, Germany.
von Hagens G; Tiedeman K; Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4): 411- 421.
https://doi.org/10.1007/BF00309677
Weber W, Henry RW. 1993: Sheet plastination of body slices - E12 technique, filling method. J Int Soc Plastination 7(1): 16-22.
https://doi.org/10.56507/EZGX2343
Weber W, Weiglein A, Latorre R, Henry RW. 2007: Polyester Plastination of Biological Tissue: P35 Technique. J Int Soc Plastination 22:50-58.
https://doi.org/10.56507/MFED4472
Weiglein AH; Henry RW. 1993: Curing (hardening, polymerization) of the polymer-Biodur S10. J Int Soc Plastination 7(1):32-35.
https://doi.org/10.56507/ABNZ7085
Weiglein AH. 1997: Plastination. A tool for teaching
https://doi.org/10.1159/isbn.978-3-8055-8714-3