1Royal Ontario Museum, Toronto, Ontario, Canada
Lincoln Memorial University, 2College of Veterinary Medicine and 3College of Osteopathic Medicine, Harrogate, Tennessee, USA
4Gubener Plastinate GmbH, Guben, Germany
A tragic mortality event for the Northwestern Atlantic population of blue whales occurred in March 2014. Two blue whale skeletons were salvaged by the Royal Ontario Museum, which also afforded the opportunity to salvage and preserve a blue whale heart. The technical challenges to preserve this heart by plastination are presented. The resultant plastinated mature blue whale heart demonstrated some variation from typical terrestrial mammalian hearts. However, this heart confirmed anatomical details of other cetacea and marine mammal species. The plastinated blue whale heart promises to be an enduring asset of tremendous scientific, educational and artistic value.
blue whale, Balaenoptera musculus, heart, plastination, preservation, massive
J.R. Miller: Royal Ontario Museum, Toronto, Ontario, Canada
![]()



Plastination has become the gold standard for preservation of anatomical specimens by replacing tissue fluid with a curable polymer. The longevity of plastinates is advantageous for preservation biological tissue, but especially rare or unique specimens of inherent scientific interest (von Hagens, 1985; von Hagens et al., 1987). Such is the case with the world’s first plastination of a blue whale heart.
Whales are iconic biological organisms, notable for their extraordinary anatomical and physiological adaptations to their obligate marine existence. The challenges and constraints imposed by this aquatic lifestyle have likely affected organ development and physiology. The blue whale is the largest of cetacean species and is the most massive animal throughout history (Small, 1971; Reeves et al., 1998; Sears and Perrin, 2018) and thus has captured scientific interest.
The blue whales, Balaenoptera musculus, can be divided into at least three subspecies and several unique populations, such as the blue whales of the North Atlantic Ocean. The excess harvest of most of the large whale species during the commercial whaling era resulted in a moratorium by the International Whaling Commission which banned commercial hunting of blue whales in the North Atlantic in 1955. Full protection of this species was extended globally by 1966 (Small, 1971; Sears and Calambokidis, 2002), along with many other whale species since then. To date, the blue whale remains critically endangered, and numbers of the Northwestern Atlantic blue whale population remain low (Sears and Calambokidis, 2002; Reilly et al., 2008; Beauchamp et al., 2009).
Limited anatomical data for larger whales has been derived from commercial whaling or from mortality events and their associated necropsies. Deceased blue whales generally sink and rarely beach (Tønnessen and Johnsen, 1982), therefore, anatomical studies are few.
In March 2014, Northwestern Atlantic blue whales experienced a tragic loss. Nine whales were found dead in the ice pack near the Cabot Strait in the Gulf of Saint Lawrence, Canada. This loss was significant, since population size is estimated only in the hundreds for this unique Northwestern Atlantic population (Sears and Calambokidis, 2002; Reilly et al., 2008; Beauchamp et al., 2009). In the spring, two dead whales drifted to the southwestern coast of Newfoundland, Canada (Allen, 2014). The Royal Ontario Museum
(ROM) obtained permits from the Canadian Department of Fisheries and Oceans to salvage skeletons from these two carcasses (permit No. NLSAR-003-14). The larger, 24.5 metre beached female was processed at Woody’s Point for a complete skeleton. The smaller, 23 metre (76 foot), mature female, processed at Rocky Harbour (Fig. 1), was partially submerged in cold water until necropsy, possibly retarding autolysis to some degree. Thus, the rare opportunity arose to salvage an intact blue whale heart.
Specimen removal
The heart of the 23 m (76 feet) mature blue whale (Fig. 1) (ROM number-125066) was collected. The vertebrae and ribs have osteoarthritic lesions which indicate an older specimen. Flensing (removal of soft tissue) of the whale carcass began caudally and proceeded cranially. Thus, abdominal cavity viscera were removed prior to thoracic cavity viscera. The heart was exposed after removal of the thoracic wall muscles (Fig. 2). With the whale in dorsal recumbency, visualization of the heart’s dorsal attachment was difficult. The great vessels (aorta and cranial and caudal vena cavae) were identified and blindly transected for removal of the heart
and lungs together. However, this unit was too large to maneuver through the intercostal space. It was necessary to separate the lungs from the heart by cutting the pulmonary vessels. Detached from the carcass and lungs, the heart was pushed out of the thoracic cavity (Fig. 2), into a nylon bag, and lifted away by a front-end loader. The heart was stored in a refrigeration truck at -1° to -2° C and transported with the skeletons to Research Casting International, Trenton, Ontario. The heart was stored at -20oC for over a year, until personnel could be assembled to proceed with cleaning, dilation and formalin-fixation. The heart weighed 175 Kg (386 pounds). An appropriate stainless tank [1.6 m x 1.75 m x 1.25 m (3,500 L)] was fabricated for thawing, dilation, fixation and storage.
Specimen preparation
In June 2015, the heart was thawed by running tap water over the heart and finally immersion in a tank with running water for 24 hours. After partial thawing, water was directed into the chambers through the transected vascular ports, to complete thawing and flush the chambers of debris (Fig. 3).
Superficial landmarks along with the great vessels were identified for heart orientation. Extraneous tissue was removed. Limited morphological measurements were taken and recorded. To flush and dilate each side of the heart (Tiedemann and von Hagens, 1982; Oostrom, 1987a; Henry et al., 1997), a cannula was secured into both atria via the cranial vena cava and a pulmonary vein. All remaining ports were occluded by securing an appropriate diameter stopper (i.e. drink bottles to 20 L buckets) into each vessel/port. Small rents were sutured. After closure of cardiac ports, tap water was directed into the submerged heart for dilation (24 h). Only partial dilation was achieved due to leakage through autolytic tissue and small lacerations, small ports and low water pressure.
For fixation, the tank and heart were transferred via a lift (Fig. 4) to a paint room with dedicated ventilation. Twenty-percent formalin solution was prepared and used to dilate the heart and displace the remaining water in the heart. 200 L of 100% formalin were diluted to ~20% to submerge and keep the heart dilating (Oostrom, 1987b). Filling, dilation and maintenance of partial dilation of right and left hearts was accomplished using two submersible pumps (1/5 HP; 1700 GPH) (Fig. 5). The buoyant heart was covered with formalin-soaked towels and a smaller submersible pump (170 GPH) was used to pump fixative over the towels to keep the surface of the heart moist. Maximal dilation of the heart could not be maintained due to
fixative leakage. For long-term, the final concentration of formalin was reduced to 15% by addition of tap water. The fixed and partially dilated heart was stored in 2300 L of 15% fixative for 5 months. A total of 600 L of 100% (37% stock solution) formaldehyde was used to provide the 20% and 15% formalin aliquots needed for fixation and storage. For the plastination venue, it was decided to utilize a working facility that was equipped to handle very large specimens and large volumes of acetone rather than to develop such a large and expensive lab for likely limited use. Gubener Plastinate, GmbH, Germany was suitable, and was chosen.
Packing and shipping
Customs offices at each border crossing were contacted in advance, to confirm customs documentation and requirements for both countries. Formalin-fixed specimen shipment [International Air Transport Association (IATA guidelines), endangered species transport, and agricultural and zoosanitation shipment requirements all had to be reconciled (CITES export permit #15CA02942/CWHQ, CITES import permit # E- 04016/15). To satisfy zoosanitation considerations, the findings of Rutala and co-workers (2008) for efficacy of formaldehyde treatment in neutralizing microorganisms, were followed.
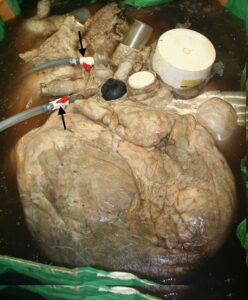
Figure 5. Heart in 20% formalin: atrial/basal view. Vessels/ports occluded. Irrigation and dilation via white cannulas (black arrows).
The heart was drained of fluid by removal of the three largest vascular port stoppers (cavae and aorta). A nylon dumpster bag was placed under the heart to raise it from the tank and the fixative solution. The tank was drained and cleaned. A wrapping protocol to comply with the IATA guidelines, included: 3 layers of poly sheet, 2-4 mm, thinnest layer next to the heart. Cellulose material (void-fill absorbent) was placed on each plastic layer. For wrapping, the heart was placed on top of this sandwich of plastic and fill. The void-fill next to the heart was saturated with water. The packaged heart was lifted back into the steel tank, which was lined with polyurethane foam sheets. Sacks of foam peanuts were placed in each corner, to create a void into which the wrapped heart would be cradled. The heart was covered with polyurethane foam and the tank sealed with silicone. Kuehn + Nagel Ltd. transported the packaged heart from Trenton, Ontario to Guben, Germany.
Vascular injection
The heart arrived in Guben, November 27, 2015 and was unpacked and inspected. The heart was dorso-ventrally flattened and had a typical formalin-fixed beige color. Multiple small cuts and imprints from packing material and stoppers were observed along with areas of arrested decomposition. The heart was transferred to a steel tank (2 m x 2 m x 1.5 m) filled with water (Fig. 4). All stoppers were removed, and the heart chambers were flushed with tap water for 24 hours.
The S14/S1 (silicone polymer [S10]/hardener [S30]) protocol was used for vascular injection (Biodur products, 2006; Coman et al., 2014). The heart was submerged in water for injection which allowed silicone leakage to float to the surface and allow visualization of defects in the vasculature. Red S14/S1-mix (80 L) was injected into the cannulated coronary arteries and 40 L of blue silicone-mix [S10, S1 (3%), AC52 (2%)] into the great cardiac vein/coronary sinus. Polymer leakage (10 L red and 20 L blue) was significant from damaged vessels (Fig. 6). After vascular injection had cured, the heart was bleached for 10 days by immersion into 5,000 L of 3% hydrogen peroxide to facilitate recolouring.
Dehydration
Gubener Plastinate GmbH’s largest vacuum chamber (4 m x 3 m x 2.2 m) was selected for two major processes of plastination: 1) dehydration with defatting and 2) impregnation (deJong and Henry, 2007). The vacuum chamber was located outdoors under cover. Since cold acetone is critical to the success of dehydration and forced impregnation (von Hagens et al., 1987), the chamber’s freezer unit afforded the required temperature of -25o C for both dehydration and impregnation. Dehydration was in a stainless steel tank nested inside the vacuum chamber. In order to stabilize and retain the natural shape of the heart during the dehydration process, an acetone resistant, low temperature resilient 1 cm thick nylon mesh was inserted as needed inside the heart and great vessels. The first phase of dehydration required five changes of cold acetone (-25o C) over 42 days. The second phase of dehydration, de-fatting in ambient temperature acetone (Tiedemann and Ivic-Matijas, 1988; Brown et al., 2002), was carried out over 4 months during the spring and summer months in Guben, Germany. To obtain ambient temperature acetone, the vacuum chamber cooling unit was shut off during this time. The dehydration process was completed on August 4, 2016. A total of 22,000 L of acetone was used during the entire dehydration process. The heart was raised and drained of excess acetone in final preparation for the next step, forced impregnation.
Impregnation
On August 4, 2016, following dehydration and draining excess acetone, the heart was submerged in 5000 L of cold (-25o C) S10/S3, silicone-catalyst mixture (100:1 ratio). Biodur AC50 red color paste was mixed into the polymer to tint the impregnation-mix as desired. Before applying vacuum, the heart was allowed to equilibrate for 2 days in the impregnation-mix. As an optional time saving procedure, residual acetone was removed from the surface of the impregnation-mix after submersion of the heart and before application of vacuum. The initial pressure applied to the vacuum chamber was lowered from ambient to 150 mbar (112 mmHg). Pressure was decreased slower after 150 mbar, until near the vapor pressure of acetone (21 mbar [~15.8 mmHg] at -25oC) when acetone began to be extracted. Vaporization of acetone was visually monitored by bubble formation. Acetone extraction/vaporization was active for 80 days.
The final pressure was 5 mbar (3.75 mm Hg) which was maintained until bubbles nearly diminished. When impregnation was complete, the vacuum was released and the heart was brought back to ambient pressure by mid-December, 2016 (Henry and Nel, 1993; de Jong and Henry, 2007). The heart was removed from the cold polymer-mix to allow surface polymer to drain freely at ambient temperature. The nylon mesh structural support was removed from the interior of the impregnated heart. The heart remained at ambient temperature for several weeks to allow for draining of excess polymer, positioning and dissection.
Positioning and dissection
Following impregnation, the malleable tissue of the heart afforded further anatomical positioning and dissection.
Positioning: The left ventricle was contoured to near diastolic conformation by using a steel armature inside the ventricle, which also served as a permanent internal scaffold. A 6 cm tube was inserted from the apex, toward and into the aorta to the origin of the brachiocephalic trunk to support the heart in a vertical orientation. This post was retained for connection to the external floor stand.
Dissection: The coronary groove was dissected to demonstrate the branches of the great vessels and their distribution. A 25 cm window was cut into the auricular surface of the right ventricle to expose the right atrio-ventricular (A-V) valve. Extraneous fascia was removed from the basal structures of the heart (great vessels and pulmonary vessels).
Curing: In preparation for curing, additional dilation of the chambers was completed by applying tension to the walls in needed directions by means of hooks and ratchets. Once in final positon, the impregnated silicone in the heart was hardened by exposure to S6 vapors. Curing at ambient temperature continued for ten days. To enhance the color of the heart for final display, and to stabilize surface detail, a mixture of S10/S3 and red color (AC50), tinted to a desired color, was brushed onto the surface of the heart. After the desired S10+S3 color-mix was created and brushed onto the heart surface, it was cured overnight with S6 vapor.
The autolytic heart (Fig. 2) of a decomposing 32 m, adult, female North Atlantic blue whale (Balaenoptera m. musculus) was successfully preserved by fixation and the Silicone S10 Standard (Cold) Procedure cold silicone plastination (Fig. 7). To appreciate the more natural aesthetics of the heart, after the first S6 cure, a colored impregnation-mix was applied to the surface, creating a pinkish hue to the once achromic formalin-fixed heart. The color was successfully set during the second and final S6 curing process.
Upon completion of the hardening process, the thick ventricular walls, aorta and pulmonary trunk were not flexible. The thinner structures: vena cavae, pulmonary vessels and atria, maintained some flexibility. The shape and conformation of the heart was preserved in a near diastolic state. External gross structures were well-preserved and observed. The internal morphology that could be seen from the limited visual vantage was also preserved. Coronary vasculature was enhanced by injecting with colored silicone polymer-mix. The heart revealed a dorso-ventral flattening, a bifid apex, and a well-defined right/left orientation of the chambers, especially on the dorsal/atrial aspect of the heart.
The final dimensions of the plastinated heart were: width: 96.3 cm and length: 106.2 cm.
The plastinated blue whale heart is an asset to ROM’s research collection and a significant element of the ROM’s travelling exhibition, “Out of the Depths: The blue whale story”. As expected, the plastinated heart is dry, non-toxic, and requires no additional preservation medium, which adds to its significance in the museum’s scientific collection. The morphology of the salvaged, autolytic blue whale heart (Fig. 2) differed from that of the typical terrestrial mammalian heart morphology: dorso-ventral flattening, bifid apex, and right/left orientation of the chambers.
The major hurdle during preservation was the heart’s large size, 175 Kg (386 pounds), along with its wet, slippery nature. Therefore, a lift and netting (Fig. 4), were needed to move the heart through each stage of the plastination process. It was difficult to turn the specimen, even when floating, to facilitate inspection of any area except that which was on the upper surface. The heart was kept moist and formalin conserved by pumping fixative over the towels covering the heart. This ensured reasonable fixation, and retarded autolysis. The autolytic heart had many open vessels which allowed excessive leakage of colored silicone while injecting the coronary vessels. The heart was submerged in water to inspect for leakage, and to keep the large amounts of leaked polymer from coating the specimen (Fig. 6). This allowed the less dense, leaked polymer to float to the surface. A heavy-duty internal support was designed to support and display the nearly 400 lb heart, and to aid in dilation.
It is interesting to note that the general principals of plastination of this large heart: specimen preparation, dehydration/defatting, impregnation and curing, were basically similar to principals for smaller more routine specimens (von Hagens, 1979a, 1979b; Oostrom, 1987a, 1987b; Tiedemann and Ivic-Matijas, 1988; Henry and Nel, 1993; Henry et al., 1997; deJong and Henry, 2007). These previous plastinated specimens have stood the test of time. Impregnation, probably one of the more critical aspects of the plastination procedure, was carried out at the same pressure, when temperature was considered, for both large and small specimens. The one prolonged variable, time of impregnation, was twice as long as for most smaller specimens. The limitation presented by the whale heart was basically volume: a matter of handling and moving the large, slippery specimen.
The unique occasion to plastinate a blue whale heart, likely the largest animal to have existed, provides an opportunity to study in detail an organ subject to several important physiological constraints. Only through an international scientific collaboration between two plastination laboratories, Lincoln Memorial University, Harrogate, TN and Gubener Plastinate GmbH, Guben, Germany, and the Royal Ontario Museum, Toronto, Canada, was this project possible. The methods described here transformed a severely-autolytic, rare specimen, into a durable specimen, while retaining accurate morphology. The project afforded insights into the challenges of plastinating very large organs, by building upon prior methodologies suitable for smaller organs (Tiedemann and von Hagens, 1982; Oostrom 1987a). This project also demonstrates that successful plastination of suboptimal material that has already undergone substantial autolysis and degradation (Fig. 2) is possible. This information should open the door and increase the potential for future recovery and preservation of rare material.
The blue whale is an iconic emblem of conservation. Opportunities to study the anatomy of this critically endangered species are rare, and every effort to preserve important anatomical material in an enduring fashion should be pursued. Preservation of the blue whale heart by plastination through cold temperature forced impregnation ensures this rare specimen will be available for study and education for a long time.
Acknowledgements
We gratefully acknowledge the generous financial support of the Louise Hawley Stone Charitable Trust, the Friends of the Canadian Collections/Amis des collections canadiennes, Royal Ontario Museum, as well as the Lincoln Memorial University: College of Veterinary Medicine and College of Osteopathic Medicine. We thank Dr. Jack Lawson and the Department of Fisheries and Oceans, Canada for assistance with permits and the procurement of the ROM's whale specimens, providing the opportunity to salvage and preserve this unique heart. We would also like to thank the staff of Research Casting International for assistance with many aspects of project logistics and early stages of preparation in Canada.
Figures 1, 5, 7, Courtesy of Lincoln Memorial University, College of Veterinary Medicine, ©LMUcvm
Figures 2, 3, 8, Courtesy of the Royal Ontario Museum, ©ROM
Figures 4, 6, Courtesy of von Hagens Plastination, ©vHP
Allen, K. 2014: Why a dead whale is so important to science. The Toronto Star, World News, Toronto, Canada. May 16, 2014.
Beauchamp J, Bouchard H, de Margerie P, Otis N, Savaria J-Y. 2009: Recovery strategy for the blue whale (Balaenoptera musculus) Northwest Atlantic population, in Canada. Species at Risk Act Recovery Strategy Series. Fisheries and Oceans Canada, Ottawa. p 62.
BIODURTM Products Catalogue. 2006. Biodur@ S14 Red. Heidelberg, Germany, p 8. http://www.biodur.de/assets/biodur_catalogue_usb_2016.pdf
Bortolloti, D. 2008: Wild Blue: A History of the World's Largest Animal. Thomas Allen Publishers, Toronto, Canada.
Brown MA, Reed RB, Henry RW. 2002: Effects of dehydration mediums and temperature on total dehydration time and tissue shrinkage. J Int Soc Plastination 17:28-33.
https://doi.org/10.56507/XNQM4606
Coman C, Enescu D.M., Iacob I, Dumitrache S, Bejinariu C., Giuglea C. 2014: Perforators of the calf arteries - anatomical study. Modern Medicine 21(3): 170-175.
De Jong, K., Henry, R.W., 2007. Silicone plastination of biological tissue: Cold-temperature technique - Biodur S10/S15 technique and products. Journal of the International Society for Plastination 22: 2-14
https://doi.org/10.56507/ZLMJ7068
Henry RW, Janick L, Henry C. 1997: Specimen preparation for Silicone Plastination. J Int Soc Plastination 12(1):13-17.
https://doi.org/10.56507/HVSK9838
Henry RW, Nel PPC. 1993: Forced impregnation for the standard S10 method. J Int Soc Plastination 7(1):27-31.
https://doi.org/10.56507/WUXP9436
Oostrom K. 1987a: Plastination of the heart. J Int Soc Plastination 1(2):12-19.
https://doi.org/10.56507/YWZL8112
Oostrom K. 1987b: Fixation of tissue for plastination: General principles. J Int Soc Plastination 1(1):3-11.
https://doi.org/10.56507/WLZH2223
Reeves RR, Clapham PJ, Brownell Jr RL, Silber GK. 1998: Recovery plan for the blue whale (Balaenoptera musculus). National Marine Fisheries Service, Silver Spring, MD. 42 pp.
Reilly, SB, Bannister JL, Best PB, Brown M, Brownell Jr. RL, Butterworth DS, Clapham PJ, Cooke J, Donovan GP, Urbán J, Zerbini AN. 2008: Balaenoptera musculus. The IUCN Red List of Threatened Species 2008: e. T2477A9447146.
https://doi.org/10.2305/IUCN.UK.2008.RLTS.T2477A9447146.en
Rutala WA, Weber DJ, and the Healthcare Infection Control Practices Advisory Committee. 2008: p 42-43, Guideline for disinfection and sterilization in healthcare facilities. Publication of Centers for Disease Control and Prevention, USA.
Sears R, Perrin W. 2018: Blue whale (Baleaenoptera musculus). In: Wërsig B, Thewissen H, Kovacs K (eds), Encyclopedia of Marine Mammals 3rd ed. Academic Press. London, San Diego, Cambridge, Oxford, p 110-113.
https://doi.org/10.1016/B978-0-12-804327-1.00070-4
Sears R, Calambokidis J. 2002: Update status report on the blue whale Balaenoptera musculus in Canada. In: COSEWIC (Committee on the Status of Endangered Wildlife in Canada) assessment and update status report on the blue whale Balaenoptera musculus in Canada. Ottawa, p 1-32.
Small, G. 1971: The Blue Whale. New York and London: Columbia University Press, p 248.
Tiedemann K, D Ivic-Matijas. 1988: Dehydration of macroscopic specimens by freeze substitution in acetone. J Int Soc Plastination 2(2):2-12.
https://doi.org/10.56507/SCLL2742
Tiedemann K, von Hagens G. 1982: The technique of heart plastination. Anat Rec 204(3):295-299.
https://doi.org/10.1002/ar.1092040315
Tønnessen JN, Johnsen AO. 1982: The History of Modern Whaling. Translated from Norwegian by RI Christonphersen. University of California Press, Berkeley and Los Angeles, 798 p.
von Hagens G. 1985: Heidelberg Plastination Folder: Collection of all technical leaflets for plastination. Anatomisches Institut I, Universitat, D-6900 Heidelberg, Germany, pp 1-10.
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4):411-421.
https://doi.org/10.1007/BF00309677