1- Department of Radiology and Nuclear Medicine Uniformed Services University of the Health Sciences
Bethesda, Maryland USA
2- Institute of Anatomy University of Heidelberg Heidelberg, West Germany
Since 1981, whole-body radiography has been performed on cadavers used in the gross anatomy course at the Uniformed Services University (1). This technique offers a unique opportunity for radiographic/anatomic/pathologic correlation and facilitates understanding of complex anatomical relationships by first-year medical students. Also, it has revealed numerous morphological variants and pathological conditions.
Heretofore, it has not been possible to retain examples of lesions or anomalous development discovered by this procedure and they were routinely cremated, along with less-interesting material. A relatively new method, called plastination (2) (3), now provides the potential for indefinite preservation of 65-70 of these specimens per year.
Principles of plastination and details of its procedures have been presented elsewhere (4). In this paper, we will demonstrate the change in imaging properties that result from plastination and discuss the teaching of radiographic correlations using plastinated specimens.
Imaging; Plastination; Silicone; S10; Biodur
Lawrence M. McNiesh Department of Radiology and Nuclear Medicine Uniformed Services University of the Health Sciences Bethesda, Maryland USA
![]()



Since 1981, whole-body radiography has been performed on cadavers used in the gross anatomy course at the
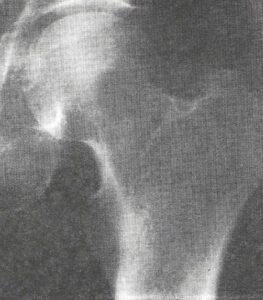
Figure la: Metastatic carcinoma of the stomach. Cadaveric AP hip shows a Grade IB lytic geographic lesion in the left femoral neck.
Uniformed Services University (1). This technique offers a unique opportunity for radiographic/anatomic/pathologic correlation and facilitates understanding of complex anatomical relationships by first-year medical students. Also, it has revealed numerous morphological variants and pathological conditions (Fig la).
Heretofore, it has not been possible to retain examples of lesions or anomalous development discovered by this procedure and they were routinely cremated, along with less-interesting material. A relatively new method, called plastination (2) (3), now provides the potential for indefinite preservation of 65-70 of these specimens per year.
Principles of plastination and details of its procedures have been presented elsewhere (4). In this paper, we will demonstrate the change in imaging properties that result from plastination and discuss the teaching of radiographic correlations using plastinated specimens.
SKELETAL IMAGING (RADIOGRAPHIC):
Routine, plain-film radiography of teaching cadavers before dissection resulted in the detection of many examples of skeletal disease. Once dissection was complete, these specimens were collected. They were then subjected to specimen radiography (Hewlett-Packard Faxitron Unit) before and after plastination with silicone rubber (SR). The trabecular pattern of pre-plastination specimens was compared with that of post-plastination specimens. Also, the occurrence of artifacts (e.g. bone dust) due to specimen preparation and plastination was recorded.
SKELETAL AND SOFT TISSUE IMAGING (MAGNETIC RESONANCE):
MR imaging of a silicone-impregnated, plastinated knee specimen was performed, utilizing the routine head coil on a 1.5T super-conducting magnet (Technicare Corp, Solon, Ohio). Five (5) mm sagittal sections with a 1 mm intervening gap were obtained, using a 256 x 256 matrix. A 700 msec TR (repetition time) and a 33 msec TE (echo delay time) were used. A multi-slice technique was used and, in all cases, the number of signal averages was two. MR images of SR-impregnated plastinated heart and brain were similarly obtained. All images were subsequently evaluated for anatomic definition.
SOFT TISSUE IMAGING (ULTRASOUND):
Tomographic ultrasound (US) images of SR- impregnated plastinated heart, brain and placenta were obtained on a real-time, dedicated breast unit with a 2.5MHz transducer. The images were evaluated for echogenicity and through-transmission.
COMPUTER-ASSISTED TOMOGRAPHIC IMAGING:
CT scans were performed on several epoxy resin (ER)- and SR- impregnated specimens, using a 4th- generation CT scanner. Axial images were compared with normal scans for anatomic detail, artifact production and tissue attenuation characteristics.
All seven SR-impregnated bone specimens showed decreased trabecular definition after plastination (Fig. 1C). Irregular medullary fat replacement was responsible for silhouetting of the trabeculae by the dense polymer. Before plastination (Fig 1b), the only artifact detected on two of these specimens was bone dust. After plastination, linear medullary lucencies, globular metaphyseal densities and soft- tissue shrinkage were noted.
MR imaging of three SR-impregnated specimens (knee, brain, heart) yielded uniformly poor anatomic definition (Fig 2). Presumably due to a dearth of mobile hydrogen ions, signal acquisition was markedly decreased, despite adequate acquisition time.
US imaging of three SR-impregnated specimens (placenta, brain, heart) was equally disappointing (Fig 3). Echogenicity could not be assessed because of the lack of through-transmission. The ultrasonic beam was strongly reflected at the incident surface of all three organs.
CT images of soft tissue and bone were degraded by shrinkage and air. Anatomic definition was variable (Fig 4a). Cortical and endosteal surfaces remained well- differentiated from adjacent soft tissue and air interfaces. Soft tissues, however, were homogeneously dense, unless permeated by air. Tissue attenuation numbers were similar for medullary space (o=578H) and soft tissue (o=574H), regardless of the polymer used (SR, ER, SR-ER copolymer) (Figs 4b and c). Nevertheless, the loss of anatomic definition noted on CT was considerably less than that seen with US and MR.
Most teachers of anatomy and pathology would agree that the use of gross tissue specimens in the laboratory is invaluable. They provide a very desirable correlate to the microscopic and radiographic study of normal and diseased structures. However useful, the preparation and handling of such material is difficult.
Plastination preserves perishable biological specimens indefinitely while greatly improving their handling qualities and durability. At present, the half-life of a conventionally stored gross pathology specimen collection at this institution is less than two years. Continuous replacement of formalin preservative is time-consuming, expensive and a health hazard. Yet, even despite such effort, specimen deterioration is inevitable.
Direct handling of formalin-stored specimens by faculty or students is impractical. Plastic display bags lose clarity very rapidly after handling. Furthermore, student manipulation quickly destroys delicate tissue or disrupts special dissections. With improved modes of therapy, exemplary cases of many classic diseases such as disseminated infection or neoplasm, have become more difficult to obtain. A more reliable means of preservation was clearly needed.
Plastinated specimens resolve many of these problems. Their potential for enhancement of the teaching of anatomy, physiology and pathology is vast (5). They can be taken into lecture and seminar sessions and simply passed around, without wetting the students' fingers or their textbooks. Also, they may be dissected, laminated or sectioned to improve information content.
For several years, the Department of Radiology/Nuclear Medicine has contributed instructional time and staff to the gross anatomy and pathology laboratory courses. Our main effort has been in the area of radiographic-anatomic and radiographic- pathologic correlation, concepts that physicians use extensively in their daily clinical activities. Plastinated specimens would be an ideal replacement for formalin-preserved material in this exercise because they are much easier to handle. However, they have not proven a practical substitute.
EFFECTS OF PLASTINATION ON IMAGE GENERATION
In the process of plastination, all tissue water and some fat is removed and replaced with plastic. The inevitable result is alteration of imaging properties. This is because radiologic resolution is ultimately dependent on differential air, fat, water and mineral content of tissues and their interfaces. Since all of the water and a significant amount of the fat is removed and replaced by an evenly distributed, dense, high-molecular-weight polymer, radiologic resolution is rendered less effective. This problem is most severe with imaging methods (US and MR) that are most dependent on tissue water for resolution.
Silicone rubbers (siloxanes) are materials consisting of high-molecular-weight molecules, each of which is a linear polymer of SiO2 with sidechains that determine special characteristics. The general siloxane formula is
where the sidechain (R) can be methyl, ethyl or oxygen. More viscous silicones are made up of longer polymeric molecules. End-to-end joining of these molecules results in the viscosity of the material. The connecting of two adjacent linear molecules by a condensation of their sidechains is called crosslinking and forms a unified matrix of the hardened polymer (6). Both of these reactions require different agents. The combination of hardening and cross-linking is called curing.
The density of cured silicone rubber effectively blocks through-transmission of an ultrasound beam (Fig 3) while its crosslinking, by markedly decreasing the number of mobile hydrogen ions, diminishes signal acquisition to a level impractical for MR (Fig 2).
CT demonstrates polymer replacement of soft tissue water and fat. It also shows the replacement of medullary fat by polymer which is of comparable density but has Hounsfield (H) numbers in the range of bone (400-600 H) (Figs 4b and c). It is therefore not surprising that silhouetting of trabecular bone by polymer was observed in plastinated bone specimens (Fig 1c). Nor is it surprising that all soft tissue structures appear uniformly dense and lose mutual differentiation.
USE OF PLASTINATED SPECIMENS IN THE TEACHING OF RADIOGRAPHIC CORRELATIONS
Despite its degrading effect on imaging, plastinated specimens retain excellent gross detail and remain histologically intact. In fact, with sheet plastination, a technique for preparing thin slices, it is possible to screen whole organs for histologic changes at considerably less expense than would be incurred with conventional methods. Areas suspected of harboring disease can then be removed from the larger screening sections and processed for conventional light or electron microscopy (7).
The altered imaging properties of plastinated specimens do not preclude their use in teaching radiographic correlations. Imaging can be performed before plastination and the plastinated specimen kept on hand for comparison. Perhaps, with further development, a polymer may be discovered that even enhances imaging.
ACKNOWLEDGEMENTS
The authors wish to thank Joseph Peters who performed the specimen radiography, Willian D. Wehunt, MD and Community Radiology Associates for specimen ultrasonography, Martha L. Ross and Shirley E. Zabrek for manuscript preparation and Gregory Holmes for specimen photography.
1. McNiesh, LM ; Madwell, JE ; Allman, Cadaver radiography in the teaching of gross anatomy. Radiology 148:73-74, 1983 https://doi.org/10.1148/radiology.148.1.6856869
2. Hagens, Impregnation of soft biological specimens with thermosetting resins and elastomers Anat Rec 194:247-256, 1979 https://doi.org/10.1002/ar.1091940206
3. Hagens, Gv. Emulsifying resins for plastination. Der Praeparator 25:43-50, 1979
4. Hagens, Gv. Heidelberg plastination folder Anatomisches Institut, University of Heidelberg, 1985
5. Bickley, H ; Hagens, Gv ; Townsend, FM. An improved method for the preservation of teaching specimens. Arch Pathol Lab Med 105:674-676, 1981
6. Manson, JA McGraw-Hill Encyclopedia of Chemistry (Parker, SR ed.) New York, McGraw-Hill Book Co, 1983
7. Tiedemann, K ; Egerer, G. Vascularization and glomerular ultrastructure in the pig mesonephros. Cell Tissue Res 238:165-175, 1984 https://doi.org/10.1007/BF00215158