1, 3 Anatomy Department, Medical College, Najran University, Najran, 55461, KSA
2Anatomy Department, College of Graduate Studies, National Ribat University, Khartoum, 12214, Sudan.
The objective of this study was to search for an economical, effective, and safe method of tissue preservation compared to the high-cost standard plastination technique currently used for preservation of human and animal tissues. This study was conducted on 144 specimens of adult sheep, divided into 11 experimental groups and one control group; each group contained 12 specimens of four halves of kidneys, hearts and brains. The experimental groups were preserved in eleven different concentrations of gum Arabic solutions, made of a mixture of gum Arabic powder, glycerine and distilled water, while the control group was preserved in silicone-S10 as the standard method of plastination used in tissues preservation. The innovative use of forced impregnation and vacuum to “infuse” the gum Arabic solution was the successful mechanism used in this new technique. It borrows the key step of the plastination technique, that is, forced impregnation to impregnate the biological tissues with gum Arabic solution. The results of the current study revealed durable, realistic preserved specimens with permanent, clear, anatomical features. In conclusion, gum Arabic solution can be used as a low-cost and safe preservation method for teaching anatomy in medical and veterinary colleges, comparable to silicone-S10 plastination, but less expensive.
gum Arabic solution, forced impregnation, preservation, kidneys, hearts, brains
Dr Mahmoud S Satte, Anatomy department, Medical College, Najran University, Najran, 55461, KSA., Telephone: +966553125185, E-mail: mahmuodsatte@yahoo.com
![]()



Preservation of tissues in their natural state, or close to the original, has helped enormously in medical and veterinary medical education. There are several methods for preservation of cadaveric and animal organs and bodies; gum Arabic and some local materials such as natron (hydrated sodium carbonate) and herbs were used traditionally by the ancient Egyptians to preserve dead bodies (Rosengarten, 1969; Abdel-Maksoud and El-Amin, 2011). Centuries later, formalin solutions have been used for fixation of tissues, however, formalin has many health hazards (Abdullahi et al., 2014). Plastination was introduced as a safe technique for preservation of cadavers by von Hagens in 1979.
In standard silicone plastination, the tissues are fixed in formalin (5 to 20%), dehydrated in acetone, impregnated in curable silicone-S10 resin and finally cured with S6 (Biodur®). The plastinated specimens were found to be more durable and odorless, showing features similar to the original, however, this procedure is relatively expensive (von Hagens et al., 1987; Grondin, 1998; De Jong and Henry, 2007).
Gum Arabic, or Acacia gum, is a natural polymer produced from wild trees of Acacia senegal or Acacia seyal which mainly grow in the African region. The chemical structure of the gum Arabic is composed largely of high molecular weight glycoprotein and polysaccharide; therefore, these components are water-soluble natural polymers (Shanmugam et al., 2005). Gum Arabic is used in food and pharmaceutical industries as an emulsifier and long-term stabilizer (Garti and Reichman, 1993). Gum Arabic solution is prepared from a mixture of gum Arabic powder, glycerine, and distilled water, which are inexpensive substances (Duaqan & Abdullah, 2013; Alkarib et al., 2015). The physical properties of gum Arabic solution, such as flexibility and viscosity, can be enhanced by the addition of plasticizing agents such as ethylene glycol, glycerine, polyethylene, and glycol (Wyasu and Okereke, 2012; Alkarib et al., 2016). To our knowledge, gum Arabic has not previously been used for preservation of biological tissues for the purpose of education in the medical field. Therefore, the main objective of this study was to assess the feasibility of using gum Arabic solution for production of affordable and safe, preserved biological tissue.
Specimen collection and fixation
A total of 72 fresh organs (24 hearts, 24 kidneys and 24 brains) of adult sheep were collected from an abattoir. The organs were transferred in an icebox to the dissection room, and then washed under running tap water to clean blood clots and fat. Each organ was cut sagittally into two halves to give a total of 144 specimens. The specimens were divided into 12 groups, with each group containing 12 specimens (4 halves of kidneys, 4 halves of hearts, and 4 halves of brains). Each group of specimens was placed in a plastic container with a tight lid, and fixed in 10% formalin for 3 days (Srisuwatanasagul et al., 2010).
Dehydration
After fixation, the specimens were dehydrated in three changes of pure acetone for 10 days at room temperature. The acetone replaces the tissues fluid and removes excess fat. The concentration of acetone of the successive changes was measured by using a hydrometer (Fisher brand, USA). When the final acetone concentration remained 99 % or above, and unchanged for a period of time, the specimens were considered dehydrated (De Jong and Henry, 2007; Elnady, 2016).
Preparation of gum Arabic solutions and curable silicone-S10
Eleven gum Arabic solutions of different concentrations were prepared from pure gum Arabic powder (Acacia senegal, Natural Gum, Sudan), distilled water and pure glycerine (Chiangrai Agro-Industry Co. Ltd., Thailand, 99.5% USP Grade). Two litres of each solution were kept in plastic containers of 3 litres capacity. The silicone-S10 (Silicones Inc., High Point, USA) was mixed with catalyst-S3 (Silicones Inc., High Point, USA) at 100:1 ratio, and was used as a control (Suganthy and Francis, 2012) (Table 1).days.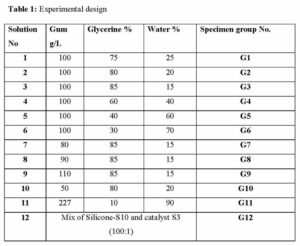
Immersion of specimens in gum Arabic solutions and curable silicone-S10
After fixation and dehydration, the first eleven groups of specimens were submerged in gum Arabic solutions, while group 12 specimens were submerged in silicone-S10/S3 mixture as shown in Table 1. The specimens were left in the different solutions for two days to equilibrate before the forced impregnation process. The submerged specimens for each group were covered by stainless steel grid to avoid the samples floating.
Intermittent forced impregnation
Forced impregnation was used for the replacement of acetone in the specimens with the gum Arabic solutions (for the experimental groups) and a curable polymer (for the control group). The submerged groups of specimens were transferred to the vacuum chamber (Mopec, USA) connected to a vacuum pump (Mopec, USA, HP200D11001) for forced impregnation at room temperature. The vacuum caused the acetone to vaporize from the specimens creating spaces in the cell for the gum Arabic solutions and polymers to diffuse into. The vacuum pressure was gradually decreased to 6 mm Hg. Vacuum was maintained for 4 days (5 hours daily) for the experimental groups and one week for the control group. Impregnation was considered completed when there were no air bubbles coming out from the specimens (Suriyaprapadilok and Withyachumnarnkul, 1997).
Post-Impregnation
After forced impregnation, the specimens were removed from the impregnation solution, and excess gum Arabic solution and polymer was allowed to drain. The specimens in each group were then arranged on a stainless steel plate for comparison and photography.
Curing
One day after forced impregnation, the control group was transferred to a closed gas curing chamber at room temperature, and cured with catalyst S6 (two times daily, 10 minutes, for three days) until the specimens were hardened (De Jong and Henry 2007). The experimental specimens were allowed to harden by atmospheric air at room temperature for one week.
Qualitative Analysis
The anatomical features of the specimens in each group were noted and recorded before and after preservation for comparison among the different groups of specimens.
The specimens preserved in gum Arabic solutions were clean, odorless and flexible. The specimens have maintained their original anatomical features and details until now, nine months after their preparation. The fine anatomical features and details of the specimens were clearly maintained in all groups preserved in gum Arabic solutions, and were similar to that of the control group, except for group 11 specimens which showed slightly unclear morphological details (Figs. 1, 2, 3).
The internal and external anatomical features that were considered for comparisons among the different groups of specimens were: the appearance of the cortex, pyramids, calyces, and pelvis of the kidneys (Figs. 1a, 1b, 1c, 1d), and the atria, ventricles, cardiac valves with attached chordae tendinae, and septa for the hearts (Figs. 2a, 2b, 2c, 2d), while the appearance of the anatomical features of the cerebral cortex, corpus callosum, thalamus, pons, cerebellum and medulla oblongata were considered for the brains (Figs. 3a, 3b, 3c, 3d, 3e, 3f).
Plastination is a modern safe technique in which polymers are used to replace tissue water and preserve tissues in a state nearest to the original form (von Hagens, 1979; von Hagens et al., 1987). The high cost of silicone resin, and the increasing demand for plastinated organs in medical and veterinary colleges encourages the search for low-cost alternative materials for the preservation of biological tissues for teaching of gross anatomy. In this study, gum Arabic solutions prepared from a mixture of gum Arabic powder, glycerine and distilled water were used for preservation of adult sheep organs. Gum Arabic is a natural agricultural product, while glycerine is an industrial by-product of soap manufacturing; both materials are safe, nontoxic, inexpensive and available in poor countries, moreover, gum Arabic solution has very good physical properties such as elasticity and viscosity (Alkarib et al., 2016).
Specimens preserved in gum Arabic solutions in the current study were realistic, odorless, dry, and flexible, and can be stored at room temperature on shelves for a long period with minimal aftercare. These facts are in agreement with silicone plastinated specimens (Pendovski et al., 2008; Suganthy and Francis, 2012).
In cold temperature plastination techniques, specimens are dehydrated at -22o C to -25o C, and this needs refrigeration (Pendovski et al., 2008; Darawiroj et al., 2010). However, in the present study, specimens were dehydrated at room temperature, which reduced the cost of purchasing deep freezers as in the cold temperature technique.
Forced impregnation at room temperature of small specimens such as porcine hearts in curable silicone-S10 was completed in more than one week (Darawiroj et al., 2010). In the present study, impregnation of the specimens in gum Arabic solution needed only 5 hours vacuum pump daily for 4 days, which indicates that gum Arabic solution needs a shorter time for the forced impregnation process, and will moreover, lead to an extended life of the plastination equipment.
De Jong and Henry (2007) mentioned that specimens are placed in a closed curing chamber, containing the cross-linking curing agent S6, for more than one week to ensure curing and hardening, however, in the current study specimens were hardened at room temperature without being cured in silicone-S6. This further reduces the cost of tissues preservation in gum Arabic solutions compared to the silicone plastination process. A
previous investigation revealed that gum Arabic solutions are not susceptible to fungal growth (Alkarib et al., 2016). In the present study, the final preserved specimens were stored on shelves at room temperature for nine months without showing any fungal growth on their surfaces.
Increasing the amount of the plasticizing agent (glycerine) in the mixture improves the elasticity and viscosity of the gum Arabic solution by decreasing the water content (Wyasu and Okereke, 2012). This coincided with the fact that the best-preserved specimens obtained in the present study were those impregnated in gum Arabic solutions with a high glycerine content (solutions 1-10) which made the preserved specimens more flexible.
Appropriate amounts of gum Arabic, glycerine, and water in the impregnation mixture are important factors that affect the final result. Hence, the best results were observed when the impregnation mixture contained less than 110 g/L gum Arabic powder, 30% to 80% of glycerine and less than 70% water. This was very obvious in group 11 specimens that had been preserved in gum Arabic solution which contained 227 g/L gum Arabic powder, 10% glycerin and 90% water (Figs. 1, 2, 3). In this group, the specimens were less flexible, and showed poor anatomical features. In general, the specimens preserved in gum Arabic solution were more flexible and less brittle (specially the brain tissues) in comparison with the silicone-plastinated specimens.
In conclusion, gum Arabic solutions can be used for production of inexpensive, safe and durable preserved specimens that can be used for teaching of gross anatomy and neuroanatomy in medical and veterinary colleges. However, further investigations are recommended about the efficiency of gum Arabic solutions for preservation of whole body and large size body specimens.
Abdel-Maksoud G, El-Amin A. 2011: A review on the materials during mummification processes in ancient Egypt. Medit Arch Archaeometry 11: 129-150.
Abdullahi M, Zagga AD, Iseh KR , Amutta SB, Aliyu D. 2014: Nasal response from formaldehyde exposure used as cadaver preservative among pre-clinical medical students in a Nigerian medical college. Int J Otol Head Neck Surg 3:173-178.
https://doi.org/10.4236/ijohns.2014.34032
Alkarib SY, Khaleel AA, Nurein MA. 2016: Gum Arabic acacia for manufacturing of hard & soft empty capsules in Sudan. World J Pharm Pharmaceutical Sci 5:219-327.
Alkarib SY, Mohamedelhassan DE, Abubakr ON. 2015: Evaluation of gum Arabic solution as a film coating former for immediate release oral tablet formulation. J Pharm Pharmaceutical Sci 5:32-41.
Darawiroj D, Adirekthaworn A, Srisuwattanasakul S, Srisuwattanasakul K. 2010: Comparative study of temperatures used in silicone impregnation of porcine hearts plastination. Thai J Vet Med 40: 433-436.
https://doi.org/10.56808/2985-1130.2262
De Jong K, Henry RW. 2007: Silicone plastination of biological tissue: cold temperature technique BiodurTM S10/S15 Technique and products. J Int Soc Plast 22: 2-14.
https://doi.org/10.56507/ZLMJ7068
Duaqan E, Abdullah A. 2013: Utilization of gum Arabic for industries and human health. Am J Appl Sci 10:1270-1279.
https://doi.org/10.3844/ajassp.2013.1270.1279
Elnady FA. 2016: The Elnady technique: an innovative, new method for tissue preservation. Altex 33: 237-242.
https://doi.org/10.14573/altex.1511091
Garti N and Reichman D. 1993: Hydrocolloids as food emulsifiers and stabilizers. Food Struct 12: 411-426.
Grondin G. 1998: Plastination: a modern approach to chiropractic teaching. J Can Chiropr Asso 42: 107-112.
Pendovski L, Petkov V, Popovska F, Ilieski V. 2008: Silicone plastination procedure for producing thin, semitransparent tissue slices: a study using the pig kidney. J Int Soc Plast 23:10-16.
https://doi.org/10.56507/JDQP3855
Rosengarten F. 1969: Ancient Egyptian and Arabian beginnings (from about 2600 BC). The Book of Spices, Jove Publ., Inc., New York. P: 23-96.
Srisuwatanasagul K, Adirekthaworn SSA, Darawiroj D. 2010: Comparative study between using acetone and absolute alcohol for dehydration in plastination procedure. Thai J Vet Med 40: 437-440.
https://doi.org/10.56808/2985-1130.2263
Shanmugam S, Manavalan R, Venkappayya D, Sundaramoorthy K, Mounnissamy VM, Hemalatha S, Ayyappan T. 2005: Natural polymers and their applications. Nat Prod Rad 4: 478-481
Suganthy G, Francis DV. 2012: Plastination using standard S10 technique - our experience in Christian Medical College, Vellore. J Anat Soc India 61: 44-47.
https://doi.org/10.1016/S0003-2778(12)80012-8
Suriyaprapadilok L, Withyachumnarnkul B. 1997: Plastination of stained sections of the human brain: comparison between different staining methods. J Int Soc Plast 12: 27-32.
https://doi.org/10.56507/YISQ6047
von Hagens G. 1979: Impregnation of soft biological specimens with thermosetting resins and elastomers. Anat Rec 194: 247-256.
https://doi.org/10.1002/ar.1091940206
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175: 411-421.
https://doi.org/10.1007/BF00309677
Wyasu G, Okereke NZJ. 2012: Improving the film forming ability of gum Arabic. J Nat Prod Plant Resour 2:314-317.