Plastination Laboratory, Center for Anatomy and Cell Biology, Medical University of Vienna, Austria
In this study, a three-dimensional (3-D) model of the pelvis was built based on thin slice plastination cross-sections of the adult female pelvis and 3-D reconstruction technology. A female pelvis was obtained, MRI scanned, plastinated, sectioned and subjected to 3-D computerized reconstruction using the WinSURF modeling system (SURFdriver Software). Qualitative observations revealed that the morphological features of the model were consistent with those displayed by typical cadaveric specimens, whilst closer morphometric analysis indicated that the model did not significantly differ from a sample of cadaveric specimens. This conclusion proves that the utilization of plastinates for generating tissue sections can be successfully integrated into 3-D computerized modeling . A better understanding of pelvic floor anatomy is relevant to gynaecologists, radiologists, surgeons, urologists, physical therapists and all professionals who take care of women with pelvic floor dysfunction. The objective of this study was to describe the method of developing this computerized model of the human female pelvis using plastinated slices. It is a method which could be applied to reconstruct any desired region of the human body.
female, pelvic floor, levator ani muscle, anatomy, plastination, 3-D reconstruction
Mircea-Constantin Sora, M.D., PhD., Centre for Anatomy and Cell Biology, Plastination and Topographic Anatomy,Währingerstr. 13/ 3 A-1090 Wien, Austria, Tel: 43 1 4277 611 50/ Fax: 43 1 4277 611 70,
E-mail: mircea-constantin.sora@meduniwien.ac.at
![]()



The pelvic floor has a complex spatial structure, knowledge of which is essential when assessing pathologies in this area (Contouris,1988; Wiliams,1995). Women, for the most part, undergo pelvic floor examinations for urinary incontinence or prolapse of the internal genitalia or of the urinary bladder (Goodrich, 1993; Lienemann, 1997). The advent of lateral urethrocystography (UCG) and colpocystourethrography has made it possible to visualize and objectively assess pathological changes in the urinary bladder and urethra, whereas pathological changes in the pelvic floor and of the supportive structures of connective tissue can at best only be evaluated indirectly. The pelvic floor muscles serve as a barrier for perianal fistulas and abscesses, and knowledge of the shape of the pelvic floor muscles, required to assess any perianal fistulas and their extent, is gained using MR imaging. The detailed anatomical and morphological information provided by sheet plastination can be added to this MR image information, which has a poorer detail resolution.
The improved properties of plastinated specimens are mainly accounted for by the superior qualities of curable polymers. In the plastination technique, tissue water and lipids are replaced by cured polymers. The class of polymer used determines the mechanical (flexible or firm) and optical (opaque or transparent) properties of the specimen. Plastinated specimens are dry, odorless, and durable; they even retain structural details down to the histological level. Today, 30 years after its introduction (v. Hagens, 1977), plastination is being applied in more than 250 departments of anatomy, pathology, forensic science and biology all over the world. In research, the technique of sheet plastination allows the arrangement of all tissue-components to be studied in their undisturbed context. This is of major interest in the borderline area between gross anatomy and histology with respect to muscular and connective tissue patterns. Epoxy resins are used to produce transparent body or organ slices, which, for research purposes, allow the study of the topography of all body structures in an uncollapsed and non-dislocated state. In addition, the specimens are useful in advanced training programs in sectional topography, resident training in CT and NMR (de Barros, 2001; Lazanoff, 2003; Thomas, 2004).
Pelvic floor dysfunction, which includes urinary and fecal incontinence as well as pelvic organ prolapse, is a highly prevalent condition in women. Ten percent of all women undergo at least one operation to treat pelvic floor dysfunction during their lifetime (Mant, 1997). The annual cost of treating urinary incontinence in Austria alone is estimated at 48 million Euros annually. However, little is known about specific pelvic floor pathomorphology and even less about pathophysiology as it relates to pelvic floor dysfunction. DeLancey et al., (2003) used Magnetic Resonance Imaging (MRI) to investigate levator ani muscle damage, and Lien et al., (2004) constructed a computerized model in order to determine the stretch forces that exceed the forces which muscle tissue can usually sustain. In our study, a three-dimensional (3-D) model of the pelvis was built for the first time based on thin slice plastination cross-sections of the adult female pelvis and 3-D reconstruction technology.
In order to investigate this topic, we need to know the orientation of the levator ani muscle and the interaction of muscles, bones, connective tissue and pelvic organs. Sections of a plastinated pelvis can help us to understand the levator ani architecture, and as the relation of muscles, fascia, organs and bones can be studied perfectly it is also well suited for a 3-D computerized reconstruction. This offers the opportunity to compare the use of identical physical and virtual models for the development of a 3-D anatomical computer model based system and its interactive manipulation.
The female human pelvis used for this study was removed from a fresh unfixed cadaver. A large specimen block, containing the pelvis, was cut into the desired sized blocks for impregnation with the maximum size of 250mm x 150mm x 150mm. High-field, high-resolution magnetic resonance imaging provides high soft-tissue contrast that allows imaging of the regular female pelvic floor anatomy to a level almost equal to plastinated macroscopic anatomic cross-sections. In this study macroscopic cadaveric cross-sections of the regular female pelvic floor anatomy are compared and correlated in-vitro with high resolution MR imaging of the same orientation. The cadaveric pelvis block was cut and adjusted to fit the standard Bruker magnetic resonance head coil (dimensions: 140x90x100 mm). Wooden markers were installed as landmarks to allow correct orientation and slice selection.
The scanned pelvis block was frozen at -80°C for one week and then plastinated following the standard ultra-thin E12 slice plastination method. (An and Zhang, 1999; Johnson, 2000; Lane, 1990; Sora, 2007 a).
Freeze substitution is the standard dehydration procedure for plastination, and shrinkage is minimized when cold acetone is used. The tissue block was placed into a -25°C freezer and then submerged in cold (-25°C) technical quality 100% acetone for dehydration. Methylene chloride was used for the degreasing and then impregnation was performed using the following epoxy mixture (Biodur E12, Rathausstr.18, 69126 Heidelberg, Germany): E12 (resin)/ E6 (hardener)/ E600 (accelerator) (Sora et al., 2007a). Once impregnation had been completed, the tissue block was removed from the vacuum chamber and inserted into a mold constructed of Styrofoam and lined with polyethylene foil. The mold containing the impregnated specimen and resin-mix was then placed in a 65°C oven for four days to harden the resin-mix. The tissue/resin block was cooled to room temperature and the mold removed. Before sawing, three colored plastic sticks were placed inside the block as markers. Using a contact point diamond blade saw, Exact 310 CP (Exact Apparatebau GmbH, Norderstedt, Germany) the hardened E12 block was cut into 1.6 ± 0.26 mm slices. Between each tissue slice, the width of the saw blade (0.4 mm), was lost. Finally the caudal surfaces of the plastinated slices were scanned into a computer using an EPSON GT-10000+ Color Image Scanner. In every scan we included a ruler as a calibration marker and used the UTHSCSA IMAGE TOOL v.2.0 for Windows software (The University of Texas Health Science Center in San Antonio) for measurements. Scanned images of the tissue slices were loaded into WinSURF (http://www.surfdriver.com) and traced from the monitor. The following features, defined as objects, were used in the reconstruction: pelvic girdle, levator ani muscle, obturator internus muscle, rectum, urinary bladder, urethra and the vagina. Each object was traced and numbered accordingly. Afterwards, the reconstruction was rendered, visualized, and qualitatively checked for surface discontinuities by rotating the model. Furthermore, WinSURF offers a measuring tool to record height, width, and depth measurements after rendering the model. Minimum requirements for the software are extremely modest and include a 200 MHz processor, Windows 2000, Windows XP, Windows 7, 24 MB of free available systems RAM (64 MB recommended), 50 MB of available disk space, 1024 x 768, 16-bit color display, CD ROM drive and 3 1/2” floppy or 100 MB Zip Drive, and a mouse or compatible pointing device; all of which are included in standard PC equipment.
Sectional plastination showed the structures of the pelvic floor muscles and their relationship to adjacent structures with a resolution down to microscopic level (Fig. 1, 2). This method allowed assessment of the course of muscle fibers. Diagnostically adequate visualization of the pelvic floor was achieved by MR imaging with a pixel size of 0.89 x 0.89 mm and soft tissue contrast.
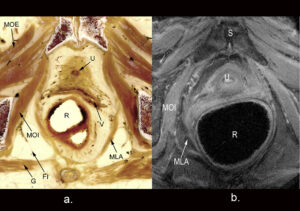 Figure 1: Transparent cross-section of the female pelvis. a. a plastinated cross-section and b. comparable MRI image. |
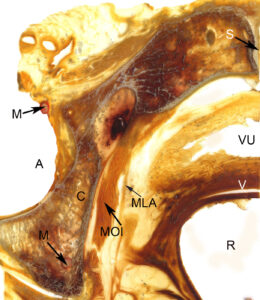 Figure 2: Plastinated cross-section at the level of the hip joint. |
By segmentation of the outlines, it was possible to create a 3-D model which consisted of the levator ani muscle, the vagina, the urethra, the rectum, the obturator internus muscles, and the pelvic bones. The anatomical structures of the pelvis could be easily identified and the borders could be traced rapidly and reliably.
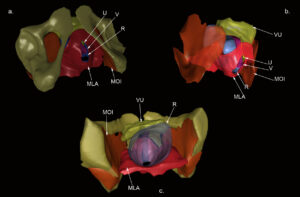
Figure 3: A 3-D reconstruction of the female pelvis. a. View of the levator ani muscle (red) from caudo-frontal. b. View from the right side with removed pelvic bones. c. View from cranial into the pelvis.
MLA – Levator ani
MOI - Obturator internus
R – Rectum
V – Vagina
U – Urethra
VU – Urinary bladder
The thin plastinated slices displayed the nerves, muscles, vessels and bones of the pelvic region distinctly . Once scanned and loaded into WinSURF, edge detection was used to quickly collect tissue borders or contours. The generated 3-D pelvis model displays a morphology corresponding qualitatively to the actual cadaver specimen (Fig. 3).
The reconstructed images appeared well-defined, especially the spatial positions and complicated relationships of contiguous structures of the female pelvis. All reconstructed structures can be displayed in groups or as a whole and interactively rotated in 3-D space. Various features such as transparency control, individual object selection, animation and a variety of manipulation modes facilitate visualization of the complex pelvic anatomy (Fig. 4).
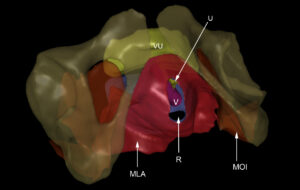
Figure 4: Front view of the 3-D reconstruction with transparent bony structures.
MLA – Levator ani
MOI – Obturator internus
R – Rectum
V – Vagina
U – Urethra
VU – Urinary bladder
The pelvic floor has a complex spatial structure, of which only parts are visualized on sectional images (Fritsch, 1995; Fritsch, 2004; Fröhlich, 1997;). It is, however, necessary to correctly relate the visualized part to the entire structure in order to properly assess pathologies. A 3-D model in which the positions of the imaging plane of interest are shown clearly improves the vividness of depiction. Additional information, such as the course of muscle fibers or connections of the muscles with each other for a given imaging plane, was gained from the plastinated sections. In contrast to anatomical preparation, the structures and spatial relationships of the tissues were not altered by plastination .
Thin plastinated slices of 1.6 mm are essential if an accurate 3-D reconstruction is desired (Qiu, 2003; Sha, 2001; Sora, 2002; Sora, 2007 b). Although the process of plastination extends the time and effort required to generate images for analysis, considerable detail is provided and the reconstructed pelvic model exhibits the bones and surrounding soft tissue. Various groups had previously investigated the female pelvic floor by computed tomography (CT), magnetic resonance (MRI) or ultrasound (US), but none had evaluated it by plastination. Transparent body slices are used for research purposes because they allow the study of body structures in a non-collapsed and non-dislocated state. Sectional plastination anatomy also confers a major advantage since the decalcification of bony tissue is not necessary, and spatial relationships are retained between contiguous features of differing composition. Thus, topography between bones and contiguous soft tissues is retained without additional chemical manipulation.
Computer models and animations of anatomical features are becoming increasingly attractive as a means to communicate complex spatial relationships and concepts effectively (Dev, 2002). Although many educational animations are based on artistic renderings (Gould, 2001), more recent applications use virtual representations derived from actual cadaveric material (Neider, 2000; Lozanoff, 2003). A logical advantage of these models is that they provide a greater sense of realism, which increases the amount of perceived information. In the future one can envision anatomy lectures consisting of only 3-D models without 2-D images (Trelease, 2002).
One of the standard interventions in the therapy of urinary incontinence is the tension-free vaginal tape (TVT) intervention (de Leval, 2003). We used the reconstructed pelvis to simulate a TVT placement (Fig. 5).
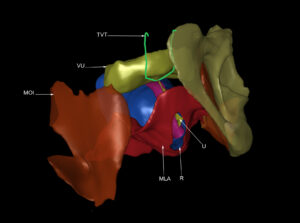
Figure 5: Reconstructed pelvis with a tension-free vaginal tape (TVT), after removal of the right side of the bony structures.
MLA – Levator ani
MOI – Obturator internus
R – Rectum
TVT – Tension-free vaginal tape
V – Vagina
U – Urethra
VU – Urinary bladder
This model will furthermore provide data to calculate stretch ratios and other biomechanical tissue properties.
The system described here relies on relatively inexpensive hardware, including a scanner and computer. The WinSURF reconstruction package, from SURFdriver Software © (surface reconstruction driver), was developed expressly for use in three-dimensional anatomical reconstruction and is a simple icon-driven system (Moody and Lozanoff, 1998).
One major problem that occurs with existing anatomical databases is the low resolution for smaller anatomical structures. Plastination can provide a useful alternative for generating anatomical databases. Moreover, plastinates are significantly easier to cut, stain, and handle compared to fresh-frozen tissue, since they are significantly more durable due to the silicone infiltrate.
Although the female pelvis reconstruction presented here did not appear to be affected by a loss of information (due to tissue loss between slices), further testing will be required to examine this issue. The capability to reconstruct individual and combined images of the pelvic structures, view them from all surgical angles, and allow for accurate measurement of their spatial relationships provides important guidance for surgeons. The reconstructed model can also be used for residency education, testing unusual surgical techniques and for the development of new surgical approaches. The 3-D model of the female pelvis presented in this paper provides a stereoscopic view to study the adjacent relationship and arrangement of respective pelvis sections.
An PC, Zhang M. 1999: A technique for Preserving the Subarachnoid Space and its Contents in a Natural State with Different Colours. J Int Soc Plastination 14: 12-17.
https://doi.org/10.56507/CQUW3856
Contouris N (1988) The human levator ani muscle. Advances in andrology. Carl Schirren Symposium, Diesbach Verlag, p 159-165.
de Barros N, Junqueira Rodrigues C, Junqueira Rodrigues A Jr, de Negri Germano MA, Guido Cerri G, 2001: The value of teaching sectional anatomy to improve CT scan interpretation. Clin Anat 14 (1):36-41.
https://doi.org/10.1002/1098-2353(200101)14:1<36::AID-CA1006>3.0.CO;2-G
DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. 2003: The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol 101(1):46-53.
https://doi.org/10.1097/00006250-200301000-00012
Dev P, Montgomery K, Senger S, Heinrichs WL, Srivastava S, Waldron K. 2002: Simulated medical learning environments on the internet. J Amer Med Informatics Assoc 9: 437-447.
https://doi.org/10.1197/jamia.M1089
Fritsch H, Hötzinger H. 1995: Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin Anat 8(1): 17-24.
https://doi.org/10.1002/ca.980080103
Fritsch H, Lienemann A, Brenner E, Ludwikowski B. 2004: Clinical anatomy of the pelvic floor. Adv Anat Embryol Cell Biol 175: III-IX, 1-64.
https://doi.org/10.1007/978-3-642-18548-9_1
Fröhlich B, Hötzinger H, Fritsch H. 1997: Tomographical anatomy of the pelvis, pelvic floor, and related structures. Clin Anat 10(4): 223-30.
https://doi.org/10.1002/(SICI)1098-2353(1997)10:4<223::AID-CA1>3.0.CO;2-T
Goodrich MA, Webb MJ, King BF, Bampton AE, Campeau NG, Riederer SJ 1993: Magnetic resonance imaging of pelvic floor relaxation: dynamic analysis and evaluation of patients before and after surgical repair. Gynecol Obstet 82: 883-891.
Gould D. 2001: The brachial plexus: developmental and assessment of a computer based learning tool. Med Educ Online 6: 1-7.
https://doi.org/10.3402/meo.v6i.4525
Johnson G, Zhang M, Barnett R. 2000: A Comparison between epoxy resin slices and histology sections in the study of spinal connective tissue structure. J Int Soc Plastination 15(1): 10-13.
https://doi.org/10.56507/CXGV7781
de Leval J. 2003: Novel surgical technique for the treatment of female stress urinary incontinence: transobturator vaginal tape inside-out. Eur Urol 44(6): 724-730.Lane A. 1990: Sectional anatomy: standardized methodology. J Int Soc Plastination 4: 16-22.
https://doi.org/10.1016/j.eururo.2003.09.003
Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. 2004: Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol 103: 31-40.
https://doi.org/10.1097/01.AOG.0000109207.22354.65
Lienemann A, Anthuber C, Baron A, Kohz P, Reiser 1997: Dynamic MR colpocystorectography assessing pelvic- floor descent. Eur Radiol 7: 1309- 1317.
https://doi.org/10.1007/s003300050294
Lozanoff S, Lozanoff B K, Sora MC, Rosenheimer J,.Keep MF, J Tregear, Saland L, Jacobs J, Saiki S, Alverson D. 2003: Anatomy and the access grid: exploiting plastinated brain sections for use in distributed medical education. Anat Rec 270: 30-37.
https://doi.org/10.1002/ar.b.10006
Mant J, Painter R, Vessey M. 1997: Epidemiology of genital prolapse: observations from the Oxford Family Planning Association study. Br J Obstet Gynaecol 104(5): 579-85.
https://doi.org/10.1111/j.1471-0528.1997.tb11536.x
Moody D, Lozanoff S, 1998: SURFdriver a practical computer program for generatingthree-dimensional models of anatomical structures using a PowerMac. Clin Anat 11: 133
Neider GL, Scott JN, Anderson MD. 2000: Using QuickTime virtual reality objects in computer-assisted instruction of gross anatomy: Yorick the VR skull. Clin Anat 13: 287-293.
https://doi.org/10.1002/1098-2353(2000)13:4<287::AID-CA9>3.0.CO;2-L
Qiu MG, Zhang SX, Liu ZJ, Tan LW, Wang YS, Deng JH, Tang ZS. 2003: Plastination and computerized 3-D reconstruction of the temporal bone. Clin Anat 16: 300-303.
https://doi.org/10.1002/ca.10076
Sha Y, Zhang SX, Liu ZJ, Tan LW, Wu XY, Wan YS, Deng JH, Tang ZS. 2001: Computerized 3-D-reconstructions of the ligaments of the lateral aspect of ankle and subtalar joints. Surg Radiol Anat 23: 111-114.
https://doi.org/10.1007/s00276-001-0111-1
Sora MC, Strobl B, Forster-Streffleur S, Staykov D. 2002: Optic nerve compression analyzed by using plastination. Surg Radiol Anat 24: 205-208.
https://doi.org/10.1007/s00276-002-0037-2
Sora M.C., 2007 a: Epoxy plastination of biological tissue: E12 ultra-thin technique. J Int Soc Plastination, 22: 40-45.
https://doi.org/10.56507/TQMH6049
Sora M.C., Genser-Strobl B., Radu J., Lozanoff S. 2007 b: Three-dimensional reconstruction of the ankle by means of ultrathin slice plastination. Clin Anat. 20(2): 196-200.
https://doi.org/10.1002/ca.20335
Trelease RB. 2002: "Anatomical informatics: millenial perspectives on a newer frontier." Anat Rec 269: 224-235.
https://doi.org/10.1002/ar.10177
Williams PL 1995: Muscles of the pelvis. In: Gray's anatomy, 38th edn. Churchill-Livingstone, London, p 831-835.
von Hagens G 1977: Patents: DBPat 27 10 147 (1978) Brit Pat 1 558 802 (1984) Brit Pat 22 082 041 (1978) Belg Pat 863.949 (1978) RSA Pat 78/1330 (1980) Austr. Pat 360 272 (1980) US Pat 4, 205.059 (1981) US Pat 4, 244, 992 (1981) US Pat 4, 278, 701 (1982) US-Pat 4, 320, 157