Division of Anatomical Sciences, Office of Medical Education, The University of Michigan Medical School, 3808 Medical Sciences II Building, Ann Arbor, MI 48109-0608, USA
This work examines the efficiency of a modified room-temperature plastination technique for preserving prenatal spinal cord morphology by assessing changes in prenatal cord length in human fetuses before and after plastination. Changes in cord length were correlated to fetal age, gender and ethnicity. Gross morphological features of the spinal cord preserved by this technique were clearly recognizable. Statistically significant shortening of spinal cords (p< 0.05) resulted when using this plastination technique. Differences in spinal cord and crown-rump lengths in relation to age were also significant. However, crown-rump length did not change significantly after plastination. Also, length changes in relation to gender and ethnicity were not significant.
human fetus; room-temperature plastination; spinal cord
Telephone: 734 -615 - 2597; Fax: 734-615-8191; E-mail: ameedr@umich.edu
![]()



The introduction of plastination as a technique for tissue preservation by Dr. Gunther von Hagens has broadened the horizon in anatomy education all over the world (von Hagens, 1987). In this process, curable polymers replace water and lipids in biological tissues. The polymer is subsequently hardened, resulting in dry, odorless and durable specimens (Weiglein, 1997). This room-temperature plastination technique is a modification of the original techniques described by von Hagens in 1978 and Glover et al. in 1998. Specimens are dehydrated in a graded acetone series at room temperature. After dehydration, specimens are immersed in liquid silicone polymer mixed with a cross linker. Vacuum is applied and when impregnation is achieved, specimens are treated with a catalyst to initiate curing.
Since its introduction four years ago, experimentation with the process continues to ensure that specimens produced are of a reasonable quality in terms of color, flexibility and clarity of detail. In search of new applications and ways to validate this technique, the spinal cord was chosen. The length of the spinal cord in human fetuses at different ages was measured before and after plastination to assess variations in cord length. The aim of this work is to examine the efficiency of another room-temperature plastination technique by demonstrating morphological features and growth changes in the spinal cord using human fetal specimens as models.
Thirty human fetuses (14 female and 16 male; 5 black and 25 white) between 16.5 and 38 weeks of age (mean = 25.2 weeks) were obtained from the Patten Embryology Collection, The University of Michigan. The fetuses were from stillbirths and miscarriages and were kept in formalin for 15 years. Spinal cords were exposed by doing laminectomies on the inferior thoracic and all lumbar vertebrae. Crown-rump (CR) and spinal cord lengths were measured. Spinal cord length was measured from the base of the skull to the tip of the conus medullaris. A pin inserted deep into the back muscles marked this level. CR length, considered here as a rough guide of the vertebral column length, was measured between the vertex of the skull to the midpoint between the apices of the buttocks as suggested by Sadler (1990). All measurements were taken pre and post dehydration and after curing.
Specimens were washed in running water for two days and then dehydrated in a graded acetone series at room temperature. Specimens were placed initially in 90% acetone for 24 hours, and then transferred to two consecutive pure acetone baths. Specimen/acetone ratio was 1:10. Dehydration was deemed complete when the concentration of acetone measured with a hydrometer was stable at 99% during the last three days. Dehydration time, five days, was the same for all the specimens.
Specimens were immersed in COR-TECH™ PR-10 silicone polymer (Corcoran laboratories) mixed with 7% cross linker (CR 22™) and vacuum was applied. Vacuum was increased slowly for 24 hours until final vacuum was achieved (28 to 29 inches of Hg). After a total of 35 hours of vacuum, no acetone bubbles were observed, impregnation was complete and vacuum was discontinued. Acetone was reclaimed in two dry ice cooled traps (Welch Vacuum #545003) (Fig. 1). About 2.8 liters of dry ice slurry (dry ice and alcohol) was used to fill each trap. Usually the holding time of the dry ice was about 4-5 hours. However, dry ice stayed for longer times when trapped acetone became less in volume during the later stages. Locally made Styrofoam® jackets to ensure better insulation and a longer holding time covered the traps. Acetone was drained from the traps through the lower port after closing the valves to maintain the vacuum while pumps were running. Trap volume was 3 liters. The level of acetone in the traps was monitored through the clear acrylic plastic lid. The initial and final volume of the polymer-cross linker reaction mixture was estimated.
Specimens were drained of excess polymer for 24 hours and then sprayed and brushed with catalyst (CT 32™). They were wrapped in plastic wrap for 6 hours, unwrapped, wiped of any excess polymer and left to cure in moist room temperature.
After curing, the spinal cord and the crown rump length of each specimen were measured. Initial measurements and those made after room temperature processing were compared. Student's t test was calculated using the SPSS statistical package.
Impregnation took 35 hours. Specimens were nearly cured and ready to handle 24 hours after the initial application of catalyst.
No gross morphological changes particularly in flexibility and firmness were observed in the fetal specimens following the impregnation and curing. General spinal cord features, e.g. the cauda equina, the dorsal and ventral roots of spinal nerves and dorsal root ganglia were clearly preserved in all specimens (Figs. 2, 3, 3a). The tip of the conus medullaris, taken as the point of spinal cord termination, was at the level of the second to third lumbar vertebra in younger fetuses and more superior (LI - L2) in fetuses approaching term. After dehydration, no
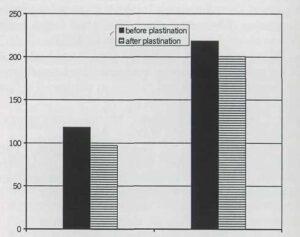
Fig. 4: Comparison of mean spinal cord and crown- rump lengths before and after room-temperature plastination.
shrinkage was measured. However, after impregnation and curing, mean length of spinal cords was 17.8% shorter than before plastination. The difference in mean length was statistically significant (p< 0.05). Also, there is a significant statistical correlation between fetal ages and spinal cord and CR mean lengths (p< 0.05). Changes in spinal cord mean length showed no significant correlation to gender and ethnicity (Table 1, Fig. 4). Crown-rump mean length did not change significantly after plastination.
Acetone collected in the dry ice-cooled traps was 12.5 liters. The volume of the polymer-cross linker reaction mixture used was 10 liters.
| N=30 | Spinal Cord Mean Length (mm) | Crown-Rump Mean Length (mm) |
| Before Plastination | 118.3 ± 32.6 | 218.5 ±62.3 |
| After Plastination | 97.2 ±25.1 | 200.5 ±51 |
| Correlation Before & After Plastination | S | NS |
| Correlation with Age | S | S |
| Correlation with Gender | NS | NS |
| Correlation with Ethnicity (B/W) | NS | NS |
Our work confirms the findings of Baker (1999) in that this room-temperature plastination technique yielded reasonable clarity and flexibility on whole and on portions of human bodies. Various gross morphological features of the spinal cord, cauda equina and dorsal and ventral roots were clearly preserved and demonstrated using this technique.
Prenatal changes in spinal cord mean lengths with age were demonstrated earlier both in animal and human models without the use of plastination (Barson, 1970; Icten et al., 1995). It is interesting that no significant decrease in cord length was observed after dehydration. The significant shortening of spinal cord mean length after curing using this room-temperature plastination technique suggests the need for further experiments. This includes, among other things, using a slower impregnation rate. Of particular interest will be the comparison of such data with those obtained using the original low-temperature as well as the original room temperature plastination techniques. Apart from the extensive research work done on the plastination of the brain (Suriyaprapadilok and Withyachumnarnku, 1997; Weiglein, 1997; Sora et al., 1999), no reports were found applying any of the plastination techniques to the human spinal cord.
Our specimens were stored in formalin for many years and most likely some degree of shortening had already taken place. It will be interesting to perform similar investigations on fresh specimens and see if the degree of shortening differs.
Our results on non-significant changes in spinal cord mean length in relation to gender and ethnicity have been reported by previous researchers (Icten et al., 1995).
This room-temperature plastination technique appears to have a promising potential for further morphological applications due to its flexibility and reliability in demonstrating different anatomical features.
Acknowledgements: The author wishes to thank Dr. Roy Glover, Director of The Plastination Lab, for his help and guidance during this work, Dr. Bruce Carlson, ex-chairman of the Department of Cell and Developmental Biology and Dr. Alphonse Burdi, Department of Cell and Developmental Biology, The University of Michigan Medical School for providing the specimens.
Baker JA. 1999: Cor-Tech PR-10 Silicone: Initial trials in plastinating human Tissue. J Int Soc Plastination 14 (2):13-19.
https://doi.org/10.56507/XVUK7879
Barson AJ. 1970: The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat 106(3):489-97.
Glover RA, Henry RW, Wade RS. 1998: Polymer Preservation Technology: POLY-CUR. A Next Generation Process for Biological Specimen Preservation. Presented at The 9th International Conference on Plastination, Trois-Rivieres, Quebec, Canada, July 5-10, 1998 J Int Soc Plastination 13(2):39.
Icten N, Memedova E, Sullu Y. 1995: Vertebral level of the ending of the spinal cord and its relationship to the length of the vertebral column in northern Turkish neonates. Surg Radiol Anat 17(4):315-8.
https://doi.org/10.1007/BF01795189
Sadler TW. 1990: Langman's Medical Embryology, Baltimore: Williams and Wilkins. p 85-95.
Sora M-C, Brugger P, Traxler, H. 1999: P40 Plastination of Human Brain Slices: Comparison between Different Immersion and Impregnation Conditions. J Int Soc Plastination 14(l):22-24.
https://doi.org/10.56507/XLSJ5724
Suriyaprapadilok N, Withyachumnarnkul B. 1997: Plastination of Stained Sections of the Human Brain: Comparison between Different Staining Methods. J Int Soc Plastination 12(l):27-32.
https://doi.org/10.56507/YISQ6047
von Hagens G, Tiedemann K, Kriz W. 1987: The current potential of plastination. Anat Embryol 175(4):411-421.
https://doi.org/10.1007/BF00309677
Weiglein AH. 1997: Plastination in the Neurosciences. AcataAnat 158:6-9.
https://doi.org/10.1159/000147902